The Impedance Cardiography Technique in Medical Diagnosis
Type of article: Review
ABSTRACT
Background: Thoracic Electrical Bioimpedance
(TEB) Technology is sometimes called Impedance Cardiography
(ICG). The Impedance Cardiography
emerged in 1940. Studies of this technique are applied to detect the
cardiovascular diseases by measuring hemodynamic parameters using skin
electrodes contact by injecting a low amplitude alternating signal. This article aims to review the various studies based on this
signal type and to present the multiple methods used for the treatment and to
have a correct analysis.
Methods: This paper is based on several
researches made in recent years published in Science Direct, Google Scholar,
and PubMed...etc. The ICG
technique consists of applying an electric field longitudinally across a
segment of the thorax with an amplitude in mean, high frequency and low
amplitude current. To analyze the ICG signal denoising is necessary; therefore, multiple filters are proposed,
and the
Discrete Wavelet Transform (DWT) denoising
is also used.
Results: The ICG is considered advantageous compared to other invasive conventional
techniques; it gives a good correlation, and solves Doppler ultrasound and Thermodilution problems. According to the studies, the Daubechies wavelet family (db8) is the best DWT to eliminate
noises. There are several algorithms for the signal characteristic point’s
detection.
Conclusion: For cardiovascular disease
diagnosis and monitoring, the non-invasive ICG technique comes to solve the
complexity problem for measurement and analyzing
heart diseases based on the thoracic electrical impedance change assessment
that is due to blood velocity and resistivity changes (blood volume changes) in
order to estimate several hemodynamic parameters.
Keywords: ICG, cardiovascular disease, hemodynamic parameters, automatic
diagnosis and monitoring, correct analysis.
Corresponding author: Hadjer Benabdallah, Department of Biomedical
Engineering, Biomedical Engineering Laboratory, Faculty of Technology,
University of Tlemcen, Algeria Email: hadjerbenabdallah@gmail.com Received: June
25, 2018, Accepted: September 02, 2018, English editing: 28
September, 2018,Published: 30 September, 2018. Screened by iThenticate.©2017-2018
KNOWLEDGE KINGDOM PUBLISHING.
1. Introduction
Transthoracic
electrical bioimpedance cardiography
or, simply, impedance cardiography (ICG) or cardiac
bioimpedance [1, 2], is based on a
theoretical model of the thorax. This technique is non-invasive, simple,
reliable, safe, painless, low cost, fast and, secure with no danger to the
subject which measures in over time the thoracic blood volume and blood
velocity variation at the aorta level due to impedance changes in each cycle.
It is used in order to extract some hemodynamic parameters that help in the cardiovascular
diseases diagnosis [3] for cardiac monitoring whether ambulatory or continuous
long-term in intensive care units (ICU). The ICG method is considered as an
alternative technique to thermodilution [4] and it is
a more advantageous technique than the conventional invasive methods. Kubicek et al. [60]
developed the four-electrode method for measuring cardiac impedance [5].
The ICG
signal can be measured by systems like BioZ, Niccomo, Osypka and Analogic [6] that
calculate the SV ejection volume, CardioScreen 2000, and
CardioScreen 1000 [7,8].
Studies
using the ICG technique are realized for patients with congestive heart
failure, with pacemakers, patients requiring fluid management, and with other conditions
[9].
Inner
electrodes measure the base thoracic impedance (Z0) during the
diastole considered constant for a patient at about 25Ω, for a man
from 20 to 33Ω and a woman
from 27 to 48Ω, [10].Pulsatile
impedance/time changes (dZ/dt), Δz and ECG
signals allow the extraction of hemodynamic parameters for the non-invasive
diagnosis of the heart and cardiac circulation to measure: (1)the stroke volume
SV; (2) the cardiac output CO; (3) the stroke volume index (SV/SVI); (4) the cardiac
index (CO/CI);(5) the left ventricular ejection time (LVET);(6) the preejection period (PEP); and (7) the heart rate (HR) among
others.
Studies of new
methods of exploration and medical treatments such as Impedance Cardiography, or ICG, emerged in 1940. In the same year,
the National Administration of Aeronautics and Space, (NASA) began the research
of the thoracic electrical bio-impedance in 1960 [11, 12] with the ICG heart
index record in a continuous, easy, non-invasive and cheaper way. The use of
this technique was recognized by the scientific and medical communities [13]. It
has been a research object since 1960, when the first test was done. In 1966
[14] the 1st impedance cardiography
monitoring device (thoracic electrical bio-impedance) was invented. In the same
year, Kubicek [5] replaced the notion of first
derivative dZ/dt usable in
the ICG method, representing the rate of the impedance variation. He tested a
systolic ejection volume (SV) equation according to the bio-impedance [15]. To measure cardiac
impedance, Kubicek et
al. [60] developed the four-electrode method [5]. In 1981,
Smarek developed a new equation in hemodynamic based
on variations in thoracic impedance [12]. In the same year, Granerus, and Elg [16] used this
signal for the left ventricular ejection volume computation, Kubicek [5] made an electrode location to estimate the
ejection volume, and Sramek [17] used 8 electrodes to
solve the problem of band electrodes to estimate ejection volume too. The 8 electrodes were
placed on the biggest part of the thorax, i.e. along the frontal plane [18].
In this
paper, the ICG signal measurement, its shape, and the different studies carried
out on this signal type are presented, as well as its characteristics which
make it possible to calculate hemodynamic parameters for the cardiovascular
diseases’ diagnosis.
2. THE ICG SIGNAL
2.1 ICG measurements
The ICG measurement is done by injecting a
low amplitude alternating signal from 0.2 mA to 5 mA and low frequency across
the current electrodes in a frequency range of 50 kHz to 100 kHz [19] and for the voltage recovery with the four-electrode
method uses four-band electrodes [20]
or 8
spot electrodes like standard ECG electrodes. The first pair of electrodes is placed
at the beginning of the thorax and the second one at the end of the thorax (the
level of the xiphoid process) [21] where the outer electrodes inject the current
and the inner electrodes measure the potential (the sensing electrodes)
(Fig.1).
The measurement is based on the skin
electrodes contact that generates impedance. In order to eliminate it, the application
of pre-gelled highly conductive electrodes is required [11]. Furthermore, the appearance of electrode-electrolyte impedance can be
greater than the impedance tested especially at low frequencies, which are too
unstable and unpredictable to think about the measurement.
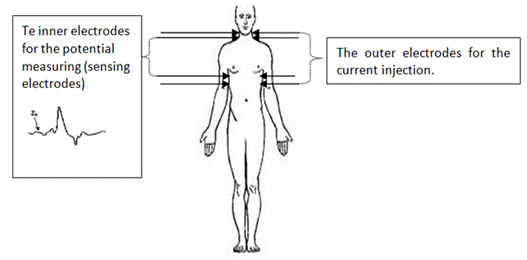
Fig.1. The
location of the 8 ICG electrodes on the human body.
2.2 The theory of hemodynamic parameters determination
a.
Stroke volume
To measure systolic time intervals based on
the bioimpedance changes in the thorax, an
alternating electric current is applying. According to Kubicek
[5], the thoracic impedance variations are due to the aorta impedance changes which
are induced by the passage of the systolic wave. An aorta segment is considered
cylindrical, and its impedance formula is as follows:
Z =![]() ,
,
where
![]() is the specific static resistance of blood;
is the specific static resistance of blood;
L: is the height of the cylinder that presents the segment of the aorta;
and
V: is the variation of blood
volume in the vessel.
When the ventricular ejection dZ/dt represents
the peaks in the acceleration time domain and (dZ/dt)max is the variation of
the trans-brachial specific resistance of the blood (Ω s-2) due to
the blood velocity variation, which represents a maximum variation rate of the
aortic volume variation, as follows [22]:
![]() max=
max=
![]() max.
max.
The systolic
ejection volume expressed in (mL / beat), which is the product of the systolic
volume and the heart rate, serves to estimate the heart health state and
extract parameters considering relevant in the diagnosis as the ejection
fraction, and it determines the CO cardiac output (approximately 70 mL /beat
for a healthy adult subject). The stroke volume equation is: SV=EDV-ESV
With
ESV as the:
end-systolic volume for a ventricle of one
person: ventricle
blood at the end of a beat; and
EDV as the:
end-diastolic volume for a ventricle of one
person: blood
before the beat.
Due to the
use of the technique of the impedance, the formulas for the SV are the
following ones [8]
[21] [24]:
According
to Nyboer [24], the volume changes in the thorax due
to the impedance variation is:
dVb= -ρb![]() or SV
= [𝜌]×
[𝐿/𝑍𝑜] 2 × Δ𝑍 .
or SV
= [𝜌]×
[𝐿/𝑍𝑜] 2 × Δ𝑍 .
According to Kubicek [5],
the equation for the systolic ejection volume depending to the thoracic impedance
variation is as follows:
SV= ρb![]() max LVET.
max LVET.
As stated by Sramek [17],
the systolic ejection volume equation depending on the thoracic impedance
variation is:
SV= ![]() max LVET,
max LVET,
where
𝜌 : is a constant
specific of the resistivity of blood and variable to person from another person
;
ρb : is the static specific resistance of blood Ω (cm)= 135 Ω cm for SV k (QUAIL et al. 1981) [23];
𝐿 : is the transthoracic
length ;
𝑍𝑜 : is the
basic impedance of the thorax (Ω); and
LVET: is the
left ventricular ejection time.
According
to D.P. Bernstein et al. [25], and
Sramek [17], the equation of systolic ejection volume
SV depending on the thoracic impedance variation and Bernstein correction
factor is as follows[15][25]:
SV= σ![]() max LVET with
max LVET with![]() ,
,
so that the other formulas have been developed and
with.
BMI as the body mass index;
δ is the Bernstein Correction
Factor;
24 is the ideal BMI value assumed by Bernstein (kg.m-2);
P: is the weight of the patient in (kg); and H is its size in (m).
The new Bernstein equation N: SV=Vc![]() max LVET,
max LVET,
where
Vc is the intrathoracic blood volume (mL).
b. The cardiac output
The
cardiac output (CO) expressed as (L / min or mL / min) is the total amount of
blood ejected by the left ventricle into the systemic circulation at each heart
beat multiplied by the heart rate in one minute, it is approximately 5.6 L /
min for the man and 4.9L / min for the woman [8]. The equations are following:
CO = stroke volume × heart rate; and
CI = cardiac output/body surface area.
2.3 ICG signal characteristics
From the
signal ICG (Fig.2), the characteristic points are extracted which allow the
calculation of the desired indices [2] [26] (Fig.3), as following:
(1) The A wave seems to coincide with the P wave of the ECG.
(2) The point B: corresponds to the opening of the
aortic and pulmonary valves. According to Lababidi et al. [27]
the point B = 15% de (dZ/dt)max. It is the point where ![]() .
.
(3) The point C:
corresponds to the maximum peak of the dZ/dt (ICG) signal on a heartbeat. It is the blood
ejection rate by the ventricles, which corresponds to the ventricular
contraction.
(4) The point X:
is the lowest point after peak C
and is associated with the closure of the aortic valve.
(5) The point Y:
corresponds to the closure of the pulmonary valve.
(6) The wave O
occurs during the diastole (the passive blood passage between the atriums and
the ventricles), its peak is the moment of the mitral valve opening.
Due to the
Pan-Tompkins algorithm, the peak C
is detected [28]. The point-by-point methods detect the points B and X from
the points C, as the manner of
the point Q detected from the
point R on the ECG. Once the
points B, C and X
have been detected, the set of cardiac indices is computable by the formulas.
In 1986, Donovan et al. showed
that if the ratio between peak O
and C (O / C> 0.3) is greater than 0.3 the patient has a pulmonary pathology [2].
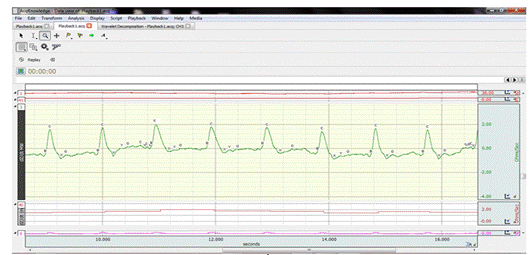
Fig.2.The shape of the ICG signal recorded on the AcqKnowledge
5.0 software.
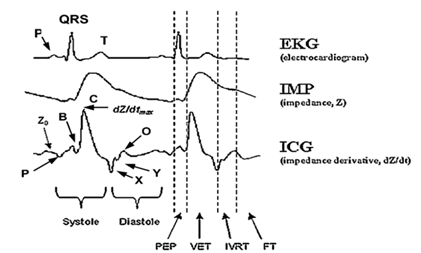
Fig.3.The composition of the ICG signal, where Z0: baseline impedance; A: atrial wave; B: aortic valve opening; C:
maximum aortic flow (dZ/dt)max;
X: aortic valve closing; Y: pulmonic valve closing; O: mitral valve opening; PEP: pre-ejection period; VET: ventricular ejection time; IVRT: isovolumic
relaxation time; and FT:
ventricular filling time [11].
3. THE ICG ANALYSIS
ICG is a diagnostic technique for cardiovascular
disease. It is used for the measurement of hemodynamic parameters that are
wrong due to noise which reaches the signal and, make the signal analysis inaccurate
and very difficult finding the correct diagnosis. The solution is to use the
wavelets to denoise the signal.
The correct
segmentation of signals makes a problem in the biomedical engineering field.
That is why many studies have been done to obtain a better approach to signal
segmentation and especially of the highly variable signals. These methods allow
creating an adequate model for a subject.
A method of
segmenting heartbeats for cardiovascular signals is based on the following
model [29]:
x (t) = Asin (2π f0 t)+ B cos (2π f0 t)+ C,
composed of 4
parameters developed by Pinheiro Eduardo et al. in 2011[30]. It is based on a
sliding power window without the need for a hypothetical formula on the shape
of patient's heart rate or a reference signal to synchronize the segmentation
points. Its
purpose is to transform the cardiac signal and obtain the fundamental heart
rate oscillation frequency. They used the ICG signal. This kind of test is done
on a wheelchair because of the artefacts due to vibration, such as movement as
well as when speaking [31]. Artefact types such as high amplitude pulses or weak
base variations cause problems of reproducibility and repeatability [32] that are eliminated through heuristic procedures [33, 34]. This method segments highly variable signals and, makes
it possible to create a suitable model for a subject, which is based on wavelet
filtering and peaks detection [33, 35].
In order to study the ICG
signal, Ermishkin relied on two hypotheses: the first
consists of heart geometry variation and that the vessels surrounded in the
pre-ejection phase, the second is the expansion of the aorta and the adjacent
arteries. He used a mathematical model based on a process summation effect with
the dZ/dt waveform and
the associated ICG parameters, the first of which refer to the WpE pre-injection wave and the second referring to the
ejection wave WEj where
ΔZ = (WpE +
WEj)
An asymmetric bell-shaped function is whose. Its form is as follows:
W (A, b, c, t) =A.e-ct.tb
He used the first and the second derivatives as well as time relations
between t0,
tmin,
and tmax
for the characteristic points
detection of the signal ICG as the
maximum of the wave C which
corresponds to (dW/dt)max and the point B which corresponds to the second
derivative [36].To
denoise the signal, in 2016 Ridha
Ben Salah [26] tested 3 types of methods described
below:(1) The term Discrete Wavelet Transform (DWT) [37] actually encompass several types of Wavelets (bases), e.g., the
Haar, Daubechies (db2, db4,
db6, and db8), Symlet (sym2, sym4, sym6, sym8), and
the Coiflet (coif2, coif3, coif4, coif5) wavelets, the
DWT equation [38] is
as follows:
X [a, b]
=![]() a,b[n]
with ѱa,b[n]=
a,b[n]
with ѱa,b[n]=![]() ,
,
where a, and b are the parameters of the wavelet
location, x[n] are the
coefficients (scaling factors), and ѱ (.) is the mother wavelet. (2) The Savitzky–Golay filter [39], and (3) The median filter.
An adaptive filtering technique based on the least
mean squares (LMS) has been proposed in [40, 41]. Meyer
wavelet-based denoising was also used for the ICG
signal [42, 43]. Ridha Ben Salah used each denoising method after measuring the C wave, which is considered the most
characteristic wave for each type, and then calculates the difference between the
C of the original signal and the C of the filtered signal. If the
difference is smaller, the method used is the more suitable for signal denoising ICG. According to the studies, the Daubechies wavelet family (db8) is the best DWT to reduce
noise, it gives a better separation between the noise and the signal. It allows
to determine the cardiovascular parameters and to diagnose the cardiovascular
diseases [26].
The study that comes next by Ben Salah et al. has an accuracy rate of 95.40%. They
worked with the normal and abnormal ICG signal for the cardiovascular diseases
detection, using a CAD system (Computer Aided Diagnosis system) for that. This
study based on (1) Temporal, (2) spectral features, and (3) classification with
the linear discriminant method [44]. The ICG technique solves the
Doppler ultrasound problem which is used to examine cardiovascular diseases as
valve heart disease (VHD) but it is expensive, and requires expertise to
perform it and discontinuity. In order to analyze the
ICG signal, in 2017, Souhir Chabchoub
[45] followed a methodology that has an
accuracy rate of 98.94%. The steps are as follows: (1) denoise
the ICG signal by the Daubechies wavelet family, (2)
segmentation where the signals will be segmented into heartbeats, (3) linear
prediction method (LP), (4) temporal and time-frequency characteristics
extraction, and (5) classification with support vector machine (SVM) and
K-nearest neighbour (KNN). The ICG was used to detect heart failure [46, 47], myocardial
infarction [48], and
mitral insufficiency [49].
4. Discussion
4.1 The evaluation of the
ICG technique
The electrical impedance has parameters
that can be used for the diagnosis and monitoring of the pathological condition
of the patient's tissues; it is measured invasively including the following
methods: (1) Direct Fick: to measure mixed oxygen concentrations of
venous blood in order to estimate cardiac output. (2) Indirect Fick: similar to the direct Fick
method, but its specificity is that it uses pulse oximetry to evaluate the
arterial oxygen content. (3) Thermodilution [50, 51]:
the temperature changes of a solution injected through the right atrial chamber
that is measured to estimate cardiac output, was distinguished by its wide
measurement variability especially in clinical practice. (4) Dye dilution: It seems like the technique
of thermodilition, based on the dye that is injected
through the pulmonary artery and it is the peripheral site that will measure
its concentration. (5) Radionuclide
angiography or ventriculography [9, 52]: allows estimating the
cardiac output by applying the dynamic sampling radioactive counts of the left
ventricle technique.
To evaluate the accuracy of the ICG
technique, the bioimpedance correlation coefficient
is calculated and compared to other techniques such as thermodilution
(TD), it is between -0.01 and 0.97. It has an accuracy comparable to
conventional invasive methods, and portability, it is easier to use, suitable
for continuous monitoring, and at low cost for many applications in cardiology [13]. The results of the
measurement are influenced by several errors such as wave positioning, patients’
weight, and pulmonary oedema [9].
Studies [22]
have been done to measure the reliability of non-invasive bio-impedance
techniques by measuring different hemodynamic parameters that are already
calculated by invasive techniques. They have chosen specific populations for
each study. Some research results are as following:
|
Deepak et
al.[54] |
TEB vs Fick A correlation rate = 0.9 |
|
Sharma et
al.[53] |
TEB vs Thermodilution
TD Results: good correlation Bland Altman with error = 19.3% |
|
Belardinelli et al.[56] |
ICG vs TD patients in the rest and the effort A correlation rate = 0.89. |
|
Cotter et
al.[55] |
TD vs ICG in a population of
patients with acute heart failure. Results: good correlation |
|
Yung GL et
al.[57] |
TD vs ICG and Fick A correlation rate = 0.8 |
|
DeMarzo AP. et al.[58] |
ICG vs Aortic Doppler The detection of the aortic valve opening by using of the ICG
technique and aortic Doppler. A high correlation rate (r = 0.996) between ICG and Doppler values. |
|
Faddy.S et al.[13] |
Database contains 27 patients with a right heart catheterization
disease. The results show a good correlation (r = 0.91) between thermodilution and TEB for the measurement of cardiac
output by using the Linear regression analysis. |
Table1. The comparison of the results from
the ICG methods with the invasive
methods for hemodynamic parameters.
4.2 The ICG technique
advantages and limitations
The ICG
technique is very useful and advantageous in the medical field because it is
non-invasive, flexible, simple, reliable, safe, painless, at low cost, manipulable by a nurse or technician, fast, it ensures the
security ( no danger on the subject) and time is saved for the care, it allows
to obtain continuous and real-time
hemodynamic data measurements as well as the diagnosis of cardiovascular
diseases such as mitral insufficiency and heart failure, but it is limited in
the field of the valvular heart disease detection [45]. It also provides a better distribution where the noises
are minimal, and the electrode-skin surface impedance is low [8]. The ICG method is affected by several changes such as:
(1) biological composition, (2) respiration, (3) noise due to movement or
equipment, (4) blood circulation, (5) volume blood from the transthoracic
region, (6) electrodes emplacement or their contacts with tissue, (7) tissue fluid
volume, (8) sweating skin, and (9) myocardial tissue contraction [59].
5. Conclusion
Impedance cardiography, or ICG, is a method to
obtain the cardiac indexes including cardiac output. This method has many
advantages that are non-invasiveness, low cost, and ease of use, but also the possible
measurements in continuity. However, it has limitations that prevent its
implementation in medical practice especially for patients’ with critical
cases. Studies are limited, clinical reports on the use of transthoracic
electrical bioimpedance cardiography
for various clinical indications in reports published from 1991 suggest that
this non-invasive method is interesting and could potentially support some of
these indications [9]. There are multiple
algorithms that are used to process ICG signals and that are not universal as
well as others that eliminate the noise and deform the signal to prevent its
correct analysis [60-63].
This article helps
readers to understand the impedance cardiography
technique, and its behaviour as well as its analysis and, to know the
hemodynamic parameters calculations theory that helps in the diagnosis of
cardiovascular diseases and their monitoring. The evaluation of this technique significantly
shows the good correlation with invasive techniques which also measure the same
parameters.
6. Conflict of interest statement
This article is an advanced version of a presentation at the
International Congress on Health Sciences and Medical Technologies 2018
ICHSMT’18.
7. Authors’ biography
Hadjer BENABDALLAH, PhD Student
Department
of Biomedical Engineering, Biomedical Engineering Laboratory, Faculty of
Technology, University of Tlemcen, Algeria.
Salim KERAI, Doctor
Department
of Biomedical Engineering, Faculty of Technology, University of Tlemcen, Algeria.
8. Reference
1.Cinca J., Warren M., Carreno A., Tresanchez M.,
Armadans L., Gomez P. Solar-Solar J.
Changes in
myocardial electrical impedance
induced by coronary
artery occlusion in
pics with and
without preconditioning:
correlation with local
ST-segment potential and ventricular
arrythmias.
Circulation 96(9), 3079-3086 (1997). Available at: https://doi.org/10.1161/01. CIR.96.9.3079
2.Woltjer H.H.,
Bogaard
H.J., de Vries P.M.J.M.The techniqueof impedancecardiography. European HeartJournal18,1396-1403(1997). https://doi.org/10.1093/oxfordjournals.eurheartj.a015464PMid:9458444
3.Shyu L.Y., Hiang
C.Y., Liu C.P., Hu W.C. (2000).Portable impedance cardiography system for real-time noninvasive cardiac
output measurement. Journal of medical and biological
Engineering,20(4),193-202.
4.Shoemaker, W. C., Wo, C. C., Chan,
L., Ramicone, E., Kamel, E.
S., Velmahos, G. C., and Belzberg, H. (2001):
'Outcome prediction of emergency patients by noninvasive hemodynamic
monitoring', Chest, 120,pp.528537. https://doi.org/10.1378/chest.120.2.528
5.Kubicek, W.G. (1966). Development
and evaluation of an impedance cardiac output system. Aerospace
Med.,37,1208-1212. PMid:5339656
6.Internet website, the
ICG Technology,
https://www.physioflow.com/sm_icg_technology.php, visited
on 12/06/2018.
7.Internet website, the
ICG systems,
https://medis.company/cms/index.php?page=icg-impedance-cardiography, visited on
05/05/2018.
8.Bera, T.K. (2014). Bioeletrical impedance methods for noninvasive health
monitoring: A review. Journalof medical
engineeringhttps://doi.org/10.1155/2014/381251
9.Jordan, H.S., Ioannidis, J.P.A.,
Goudas, L.C., Chung, M., Kupelnick, B., Miller, K., Terrin, N., Lau J., Thoracic Electrical Bioimpedance
[Internet], Rockville (MD): Agency for Healthcare Research and Quality (US);
2002 Nov 27.).
10.Stevanovic,P.,
Scepanovic, R.,Radovanovic,D.,
Bajec, D., Perunovic, R., Stojanovic, D., Stevnovic, D.
(2008). Thoracic Electrical Bioimpedance Theory and
Clinical Possibilities in Perioperative Medicine, Signa
Vitae: journal of intensive care and emergency medicine, 3(suppl.1) 22-27.
11.Summers, R.L., Shoemaker, W.C.,
Peacock, W.F., Ander, D.S., & Coleman, T.G. (2003). Bench to bedside: electrophysiologic and clinical principles for noninvasive
hemodynamic monitoring using impedance cardiography.Academic
emergency medicine,10(6),669-680. https://doi.org/10.1197/aemj.10.6.669
https://doi.org/10.1111/j.1553-2712.2003.tb00054.x PMid:12782531
12.Bayod S., Hermant, A. Les
applications de la bioimpédance, Projet DESS, UTC,
98-99, pp 53.
13.Faddy, S., Boland, J. &
Muller, D.W.M. (2003).Accuracy and reliability of
non-invasive cardiac output:the future in
cardiology?. In Computers in cardiologie,2003(pp.251-253).IEEE.
https://doi.org/10.1109/CIC.2003.1291138
14.Shoemaker, W.C., Wo, C.C., Bishop,
M.H., Appel, P.L., de Water Van, J.M., Harrington,G.R.& Patil, R.S. (1994).
Multicenter trial of a new thoracic electrical bioimpedance
device for cardiac output estimation. Critical care medicine, 22(12),1907-1912.https://doi.org/10.1097/00003246-199412000-00004PMid:7988125
15.Tsadok, S. (1999).The
historical evolution of bioimpedance. AACN Advanced
Critical Care, 10(3),371-384.
16.Granerus, G., &Elg, R.(1982).Stroke Volume
Measurement by Impedance Cardiography Using a Formula
Based on the ∆z Waveform. Clinical Physics and Physiological Measurement,
3(2),131. https://doi.org/10.1088/0143-0815/3/2/003PMid:6126290
17.Sramek B.B. (1986) BoMed's electrical bioimpedance
technology for thoracic applications (NCCOM): Status report, May 1986 Update.
Irvine, BoMed Ltd, 1986, 19+2 p.
18.Choudhari, P. &Panse,M.S.
(2013). Measurement of Cardiac Output using Bioimpedance
Method. In IJCA Proceedings on International Conference on Communication
Technology ICCT(vol.2,pp.28-33).
19.Shih, H., & Lo, T.C. (1996).
Electrochemical impedance spectroscopy for battery research and development. Cortech Corporation. CA, Tech.Rep,
31(9-11) .
20.Huysmans, M. C .,
Longbottom, C., Bitts, N.B., Los, P., & Bruce,
P.G. (1996). Impedance spectroscopy of teeth with and without approximal caries lesions- an
vitro study, Journal of dental research,75(11),1871-1878. https://doi.org/10.1177/00220345960750110901PMid:9003234
21.Malmivuo,P.,
Malmivuo, J. & Plonsey,
R.(1995). Bioelectromagnetism: Principles and
Applications of Bioelectric and Biomagnetic Fields.
Oxford University Press, USA. https://doi.org/10.1093/acprof:oso/9780195058239.001.0001PMid:7494216
22.Panse,P.,&Choudhari,M.S (2013). Measurement of Cardiac Output using Bioimpedance Method. International Journal of Computer
Applications, 28-33.
23.Quail, A. W., Traugott,
F. M., Porges, W. L., and White, S. W. (1981):
'Thoracic resistivity for stroke volume determination in impedance cardiography', J. Appl. Physiol., SO, pp. 191 195.
24.Internet Article, Overview of Impedance
Cardiography (ICG), http://impedancecardiography.com/icgover10.html
25.Bernstein, D.P., Lemmens, H.J.M. (2005) Stroke Volume Equation for Impedance
Cardiography. Medical and Biological Engineering and
Computing, 43(4), 443-450. https://doi.org/10.1007/BF02344724
26.Chabchoub, S.,Mansouri, S., & Salah, R.B. (2016). Impedance cardiography signal denoising
using discrete wavelet transform. Australasian physical & engineering
science in medicine, 39(3), 655-663. Available at: DOI
10.1007/s13246-016-0460-z. https://doi.org/10.1007/s13246-016-0460-zPMid:27376722
27.Lababidi Z, Ehmke DA, Durnin RE, Leaverton PE, Lauer RM
(1970). The first derivative thoracic impedance cardiogram, American Heart
Association. Circulation 41:651–658 https://doi.org/10.1161/01.CIR.41.4.651PMid:5437409
28.Pan, J., Tompkins, W.J. (1985). A
real-time QRS detection algorithm. IEEE Transactions on Biomedical Engineering,(3):230–236. https://doi.org/10.1109/TBME.1985.325532PMid:3997178
29.Ramos, P.M., & Serra, A. C.
(2008). Impedance measurement with
sine-fitting algorithms implemented in a DSP portable device. IEEE Transactions
on instrumentation and measurement,57(1), 197-204 https://doi.org/10.1109/TIM.2007.908276
30.Pinheiro, E., Postolache,
O.,& Girao, P. (2011). Method for segmentation of
cardiac signals based on four parameter sine fitting. In EUROCON-International
Conference on Computer as a Tool (EUROCON°, 2011 IEEE(pp.
1-4). IEEE. https://doi.org/10.1109/EUROCON.2011.5929306
31.Pinheiro,E.C.,
Postolache, O.A. &Girao,
P.S. (2010). Automatic wavelet detrending benefits to
the analysis of cardiac signals acquired in a moving wheelchair. In engineering
in Medicine and biology Society (EMBC), 2010 Annual International Conference of
the IEEE, pp.602-605).IEEE. https://doi.org/10.1109/IEMBS.2010.5626646PMid:21096105
32.Pinheiro,E.,Postolache,O.,
& Girao,P.(2010). Theory and developments in
an unobtrusive cardiovascular system representation: Ballistocardiography.
The Open Biomedical Engineering Journal, 4,201.
https://doi.org/10.2174/1874120701004010201PMid:21673836 PMCid:PMC3111731
33.Zhu, X.,Chen,W.,Nemoto,T.,Kanemitsu,Y.,Kitamura,K.I.,Yamakoshi,K.I., &Wei, D. (2006). Real-time monitoring
of respiration rhythm and pulse rate during sleep. IEEE transactions on
biomedical engineering, 53(12), 2553-2563. https://doi.org/10.1109/TBME.2006.884641PMid:17153213
34.Shin, J.H.,Choi,B.H.,Lim,Y.G.,Jeong,D.U. &Park, K.S. (2008). Automatic ballistocardiogram (BCG) beat detection using a template
matching approach. In Engineering in Medicine and Biology Society,
2008. EMBS 2008. 30th Annual
International Conference of the IEEE (pp.1144-1146). IEEE. https://doi.org/10.1109/IEMBS.2008.4649363PMid:19162866
35.Postolache, O.,Girao, P.S.,Postolache,
G., & Pereira, M.(2007). Vital signs monitoring system based
on emfi sensors wavelet analysis. In instrumentation
and Measurement Technology Conference Proceeding, 2007. IMTC 2007. IEEE, pp.
1-4).IEEE. https://doi.org/10.1109/IMTC.2007.378999
36.Ermishkin, V. V., Kolesnikov,
V.A., Lukoshkova, E.V., &Sonina,
R. S (2013). Simulation of 'pathologic'changes
in ICG waveforms resulting from superposition of the 'preejection'
and ejection waves induced by left ventricular contraction. In Journal of
Physics: Conference Series(Vol. 434, No. 1, p.012007).
IOP Publishing. https://doi.org/10.1088/1742-6596/434/1/012007
37.Addison PS (2005) Wavelet
transforms and the ECG: A review. PhysiolMeas
26:R155–R199 https://doi.org/10.1088/0967-3334/26/5/R01
38.Cohen, L. (1989). Time-frequency
distributions—A review. Proceedings of the IEEE, 77(7):941–981. https://doi.org/10.1109/5.30749
39.Luo,J.,
Ying, K., He, P., Bai, J. (2005). Properties of Savitzky-Golay
digital differentiators. Digit Signal Processing, 15(2) 122–136. https://doi.org/10.1016/j.dsp.2004.09.008
40.Pandey,V.K.,
Pandey, P.C., Burkule, N.J., &Subramanyan,
L.R. (2011). Adaptive filtering for suppression of respiratory artifact in
impedance cardiography. In Engineering in Medicine
and Biology Society, EMBC 2011 Annual International Conference of the IEEE
(pp.7932-7936). IEEE. https://doi.org/10.1109/IEMBS.2011.6091956PMid:22256180
41.Hu, X., Chen, X., Ren, R., Zhou,
B., Qian, Y., Li, H., Xia, S. (2014). Adaptive filtering and characteristics
extraction for impedance cardiography. Journal of
Fiber bioengineering and Informatics,7(1),81-90.
42.Pandey, V.K., Pandey, P.C. (2007).
Wavelet based cancellation of respiratory artifacts in impedance cardiography. In Digital signal Proceedings, 2007 15th
international conference on (pp.191-194).IEEE. https://doi.org/10.1109/ICDSP.2007.4288551
43.Pandey, V.K., Pandey, P.C. (2009).
Wavelet based denoising for suppression of motion
artifacts in impedance cardiography. In: Proceedings
of the international symposium on emerging areas in biotechnology &
bioengineering. Mumbai.
44.Salah, R.B., Alhadidi, T., Mansouri, S. & Naouar, M.
(2015). A new method for cardiac diseases
diagnosis. Advances in bioscience and biotechnology, 6(04), 311. https://doi.org/10.4236/abb.2015.64030
45.Chabchoub, S., Mansouri,S., & Salah, R.
B. (2017). Detection of valvular heart diseases using
impedance cardiograpgy ICG. Biocybernetics
and Biomedical Engineering. Available at:
https://doi.org/10.1016/j.bbe.2017.12.002. https://doi.org/10.1016/j.bbe.2017.12.002
46.Khraim, F., Pike, R., Williams, J.
(2014) Using non invasive impedance cardiography to assess cardiac hemodynamic measures of
persons with heart failure. Canadian Journal of Cardiology,30(10)S371. https://doi.org/10.1016/j.cjca.2014.07.708
47.Packer, M., Abraham, W.T., Mehra, M.R., Yancy,C.W., Lawless, C.E., Mitchell J.E., Pina, I.L. (2006). Utility of
impedance cardiography for the identification of
short-term risk of clinical decompensation in stable patients with chronic
heart failure. Journal of the American College of Cardiology, 47(11),2245–2252.
https://doi.org/10.1016/j.jacc.2005.12.071PMid:16750691
48.Chen, S.J., Gong, Z., Duan, Q.L. (2014). Evaluation of heart function with
impedance cardiography in acute myocardial infarction
patients. International journal of clinical and experimental medicine,
7(3),719. PMid:24753769 PMCid:PMC3992414
49.Chabchoub, S., Mansouri,
S. & Salah R.B. (2016).Diagnosis of mitral
insufficiency using impedance cardiography technique
ICG. Journal of Electrical Bioimpedance, 7(1),28-34. https://doi.org/10.5617/jeb.2872
50.Bogaard, H.J., Woltjer,
H.H., Postmus, P.E.,& de
Vries, P.M.J.M. (1997). Assessment of the Haemodynamic Response to Exercise by Means of Electrical
Impedance Cardiography: Method, Validation and
Clinical Applications. Physiological Measurement, 18(2),95. https://doi.org/10.1088/0967-3334/18/2/001PMid:9183804
51.Jansen, J.R.C.(1995).The
thermodilution method for the clinical assessment of
cardiac output. Intensive Care Medicine, 21(8),691-697. https://doi.org/10.1007/BF01711553PMid:8522677
52.Espersen,K.,
Jensen, E.W., Rosenborg, D., Thomsen, J.K., Eliasen, K., Olsen, N.V.,&Kanstrup,
I.L.(1995). Comparison of cardiac output measurement techniques: thermodilution, Doppler, CO2-rebreathing and the direct
Fick method. Acta Anaesthesiologica,
Scandinavica,39(2),245–251.
https://doi.org/10.1111/j.1399-6576.1995.tb04051.xPMid:7793193
53.Sharma,V,
Singh, A., Kansara, B. &Karlekar,
A. (2011).Comparison of transthoracic electrical Bioimpedance
cardiac output measurement with thermodilution method
in post coronary artery bypass graft patients. Annals of cardiac
anaesthesia,14(2),104. https://doi.org/10.4103/0971-9784.81564PMid:21636930
54.Barde, P., Bhatnagar,
A., Narang,R.,& Deepak, K.K. (2012).Comparison of non-invasive
cardiac output measurement using Indigenous impedance cardiography
with invasive Fick method. International Journal of Biomedical
Research,3(11).https://doi.org/10.7439/ijbar.v3i11.816
55.Cotter, G., Moshkovitz,
Y., Kaluski,E.,Cohen,A.J.,Miller,
H., Goor, D., &Vred, Z.
(2004). Accurate, noninvasive continuous monitoring of cardiac output by
whole-body electrical bioimpedance. Chest,
125(4),1431-40. https://doi.org/10.1378/chest.125.4.1431PMid:15078756
56.Belardinelli, R., Ciampani, N., Costantini, C.,Blandini, A.,&Purcaro, A.(1996). Comparison of impedance cardiography with thermodilution
and direct Fick methods for noninvasive measurement of stroke volume and
cardiac output during incremental exercise in patients with ischemic
cardiomyopathy. The American Journal of Cardiology,77(15),1293-1301. https://doi.org/10.1016/S0002-9149(97)89153-9.
57.Yung, G.L., Fedullo,
P.F., Kinninger, K., Johnson, W. &Channick, R.N. (2004). Comparison of impedance cardiography to direct Fick and thermodilution
cardiac output determination in pulmonary arterial hypertension. Congest Heart
Failure,10(s2),7-10. https://doi.org/10.1111/j.1527-5299.2004.03406.x
58.DeMarzo, A.P. ,
Lang, R.M. (1996).A New Algorithm for Improved Detection of Aortic Valve
Opening by Impedance Cardiography. In Computers in
Cardiology ,1996 (pp. 373-376). IEEE. https://doi.org/10.1109/CIC.1996.542551
59.Funk, D.J., Moretti, E.W., & Gan, T.J. (2009). Minimally invasive cardiac output
monitoring in the perioperative setting. Anesthesia&
Analgesia,108(3),887-897. https://doi.org/10.1213/ane.0b013e31818ffd99PMid:19224798
60.Kubiček, W. G., Kottke, J., Ramos, M. U., Patterson, R.P., Witsoe, D.A., Labree,J.W. &Garamela, J.T.(1974).The
Minnesota impedance cardiograph-theory and applications. In Bio-Medical
Engineering.
61.Allen, M.T., Fahrenberg,
J., Kelsey, R.M., Lovallo, W.R. &Doornen, L.J.(1990).Methodological
guidelines for impedance cardiography.
Psychophysiology, 27(1), 1-23 https://doi.org/10.1111/j.1469-8986.1990.tb02171.xPMid:2187214
62.Lozano, D. L., Norman, G., Knox, D.,Wood,B.L.,Miller, B.D., Emery, C.F.,&Berntson, G. G. (2007).Where to B in dZ/dt.Psychophysiology,44(1),113-119.Available
at: https://doi.org/10.1111/j.1469-8986.2006.00468.x
63.Ono T, Miyamura
M, Yasuda Y., Ito,T., Saito,T., Ishiguro,
T. &Yambe, T.(2004). Beat-to-beat evaluation of
systolic time intervals during bicycle excercise
using impedance. Tohoku J.EXp.Med,203,17-29.Available at:
https://doi.org/10.1620/tjem.203.17