Are the plants used in Algerian traditional medicine effective?
Assessment of the antibacterial, anti-inflammatory and
anti-oxidative effects of three plants used in Algerian traditional Medicine;
Olea europaea, Glycyrrhiza glabra and Ocimum basilicum.
Type of article: Original
Elkolli Meriem, Elkolli Hayet
Department of microbiology, FSNV of Setif 1
university, Algeria
Abstract
Background: Algeria has a very large vegetation biodiversity. Algerians use herbs
in phytotherapy because of their easy, safe and inexpensive use. However, the
consumption of these plants remains uncontrolled or regulated by the
authorities, which lacks reassurances concerning their use.
Objective: The purpose of this work is to confirm or refute the empirical use of
the plants as well as to start adjusting the dosages for each therapeutic
purpose.
Methods: The present experimentation was done during the year 2017 (April-June)
at the laboratory for the valorization of natural biological substances at the
University of Setif 1. The in vitro anti-inflammatory effect of Olive and basil
leaves and Licorice root aqueous extracts were evaluated against Escherichia
coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus
ATCC 25923 by the disc diffusion assay. The in vitro anti-inflammatory activity
was realized by the estimation of the protein denaturation of the BSA, and the
anti-oxidative test was done by the DPPH method. All experiments were done in
triplicate results and were reported as mean ± SD. Data were analyzed by
GraphPad prism 5 software; the statistical analysis was done by the one way
ANOVA.
Results: Olive leaf extract (OLE) and Licorice root extract (LRE) were active on
both E. coli (13.5, 10mm) and S. aureus (14, 12mm) at 200 mg/ml. While the
Basil leaf extract (BLE) was inactive against all strains. The percentage of
BSA denaturation was concentration-dependent by both BLE and LRE and the
maximum inhibition was recorded by the OLE at 250 μg/ml, it was slightly
different from BLE at P≤0.05, but not significantly different from LRE. The
three extracts showed good values of IC50 with 0.65, 4.98 and 0.91 mg/ml OLE,
LRE and BLE respectively, but they were inferior to that of BHT.
Conclusion: These results confirm the use of these plants but under control.
Keywords: traditional phytotherapy, Olive leaves, Licorice, Basil.
Corresponding
author: Elkolli Meriem, Department
of microbiology, FSNV of Sétif 1 university Algeria elkollim@yahoo.fr
Received: 11 July, 2018, Accepted: 14 July, 2019, Published: 20 October,
2019.
Screened by iThenticate..©2017-2019 KNOWLEDGE KINGDOM PUBLISHING.
1.
Introduction
In Algeria, the pharmaceutical market is
growing rapidly, as Algeria intends to develop local production and become a
national production platform, knowing that a large share of the market is based
on imports (close to 70 %) (Bouzabata, 2016). Often, the customer is attracted
by the personality of the herbalist. Indeed, some herbalists express themselves
perfectly, in the three languages, Arabic, Berber and French. They have the
assurance of the therapist, do not hesitate to make reference to the
international books (of Europe, America or the Middle East) and give examples
lived by their clients, they give orally true prescriptions, with dosage,
duration of treatment and administration mode (Hammiche et al., 2013). The
problem is that, recently, there is a decrease in the knowledge of medicinal
plants in young generations with a growth in the number of herbalists not
specialized in this field. In order to establish the list of medicinal plants,
which are sold at the local market and which really have biological effects
that corroborate the use, we tested three of these plants for their antibacterial,
anti-oxidative and anti-inflammatory activities: Olive and Basil leaves and
Licorice roots. The Algerians consider the Olive tree (Olea europaea/
Oleaceae), known locally as “Zitoun”, as an almost sacred tree because of its
multiple virtues; it is even evoked in the holy Quran. The Olive tree is one of
the most typical and economically important tree in the Mediterranean countries
and its leaves are one of the byproducts that can be found in high amount (Orak
et al., 2012). Its leaves and barks have astringent, diuretic, febrifuge,
hypoglycemic, tonic and hypotensive properties. Traditionally, Olive leaf
infusion is used as a gargle against oral conditions (inflammation of the gums,
canker sores and bad breath) (Rebas et al., 2012). Common Basil, named locally
“H’bak”, (Ocimum basilicum/ Labiatae) is grown all over the world, in the warm
and temperate zones, due to its great popularity. It is an important economic
and medicinal herb. The fresh leaves are used as flavourings and have been used
as an appetite stimulant, carminative, diuretic, mouth wash and astringent to
cure inflammations in the mouth and throat (Hiltunen and Holm, 1999). Licorice
(Glycyrrhiza glabra/ Fabaceae), locally called “Erg Essous”, is native to
Eurasia, in central and south-western Asia and the Mediterranean. It is used
fresh or dried as a mouth freshener, tooth cleaner, expectorant and
carminative, flavoring agent, antimicrobial, antioxidant and anti‐inflammatory (Lim, 2016; Rohinishree and Negi,
2016). This work is part of an ethnopharmacological survey that aims to
standardize the sale of medicinal plants by herbalists. In this study, an
assessment of biological activities of these three plants, sold for their
anti-infectious and anti-inflammatory properties, was done.
2.
Materials
and Methods
2.1.
Plant material
Olive (Fig.1,a) and basil leaves (Fig.1,b) and
licorice roots (Fig.1,c) were used to prepare the aqueous extract (Aqe). The
three plants were procured from a local herbalist in Sétif. The choice of
plants was made according to a survey done beforehand.

Figure 1: Olive leaves (a), basil leaves (b) and licorice roots (c)
2.2.
Bacterial strains, culture media and chemical reagents
The antibacterial activity was evaluated on
three bacterial strains of the American Type Culture Collection (ATCC):
Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and
Staphylococcus aureus ATCC 25923. Muller-Hinton agar medium (MHA), in addition
to various chemical reagents, namely methanol, 2,2'-diphenyl-1-picryl hydrazyl
(DPPH), dimethyl sulfoxide (DMSO), Butylated hydroxytoluene (BHT), bovine serum
albumin (BSA), Aspirin and Gentamicin (GM) discs. These reagents come from
different sources; Sigma, Fluka, Prolabo and Sanofi Diagnostic.
2.3.
Preparation of the aqueous extract
The
choice of the aqueous extract was made in accordance with the use. Practically,
20g of dried Olive leaves 20g of dried leaves of Basil and 40g of root of
Licorice were each placed in 500ml of cold water. The mixtures were boiled for
15, 5, and 25 min respectively. Subsequently, the mixtures were filtered and
subjected to ambient air drying to have the aqueous extract. The extraction’s
yields are calculated as following:
![]()
2.4.
Agar Diffusion Method
Discs of six mm diameter filter paper,
impregnated with different concentrations (50, 100, 150, 200 mg/ml) of the Aqe,
are deposited on MHA inoculated with a standardized inoculum at 0.5 Mc Farland
density. Gentamicin is tested in simultaneously. The Petri dishes are then
incubated at 37 ° C. for 24 hours. After incubation, the diameters of the
inhibition zones are measured around each disc (Rahal et al., 2008). To
determine if there a bactericidal or bacteriostatic effect, a sample from the
inhibition zone is transferred to a Petri dish containing MHA and is incubated
at 37°C for 24 h and then examined with the naked eye. Bacterial growth indicates
a bacteriostatic effect, while an absence of growth indicates a bactericidal
effect of the extract tested.
2.5. In-vitro anti-inflammatory activity
Inhibition
of protein denaturation is evaluated by the method of (Reshma et al.
2014)
with slight modification. 500 μl of 1% BSA are added to 100 μl of plant extract
with different concentrations (250, 500, 1000 µg/ml). This mixture is kept at
room temperature for 10 minutes, followed by heating at 51°C for 20 minutes.
The resulting solution is cooled down to room temperature and absorbance is
recorded at 660 nm. Acetyl salicylic acid is taken as a positive control. The
experiment is carried out in triplicates and percent inhibition for protein
denaturation is calculated as follows:
![]()
Where: As is the absorbance of the sample, Ap is the
absorbance of the product control and Ac is the absorbance of the positive
control.
2.6.
DPPH free radical scavenging assay
The DPPH radical absorbs at 517nm and the antioxidative
activity can be measured by monitoring the decrease in this absorbance. The
method used is that of (Singh et al., 2006) slightly modified. Practically 50
µl aqueous extract of each plant with different concentrations are mixed with
1250 µl of 0.004% methanolic solution of DPPH. The absorbance is measured at
517 nm after 30 min of incubation in the dark. BHT is used as a standard and is
subjected to the same treatment. The DPPH scavenging activity is calculated as
following:

Where At is the absorbance of the test, Ac is
the absorbance of the control.
2.7.
Statistical analysis
Values are expressed as mean ± Sd. The results
of the various tests are analyzed by the univariate ANOVA followed by the
Dunnett and Tukey tests for multiple comparisons and determination of
significance values. The statistics are made by Graphpad Prism 5
3.
Results
and discussion
3.1.
The extraction yield
The
yield obtained by decoction of Basil (23.14 ± 1.99%) was the highest compared
to the other plants; Olive leaves (5.82 ± 0.09%) and Licorice (5.29 ± 4.17%) (Table1).
Table 1:
The yield, color and
appearance of the extracts obtained

3.2.
Antibacterial assay
Olive leaves (OLE) and Licorice roots extracts (LRE) were active on E. coli (13.5, 10mm) and S. aureus (14, 12mm) respectively at the highest concentration (200mg / ml) with total resistance of P. aeruginosa (which is known to be resistant to many antibacterial agents) (Table 2).
Table 2:
inhibition diameters in mm: each value represents 3 measures ± SD
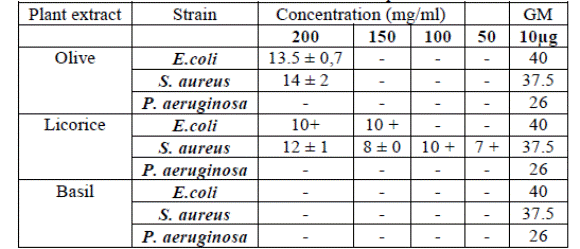
-; no
activity, +: decrease in bacterial density, GM: gentamycin.
3.3. Anti-inflammatory assay
The data of our study show that the maximum
percentage of inhibition was observed from OLE, at 250 μg/ml, slightly different (P ≤
0.05) from BLE, but not significantly
different from LRE (Fig.2). There
is also a concentration-dependent inhibition of protein (albumin) denaturation
by both BLE and LRE throughout the concentration range of 250 to 1000 μg/ml
with a non-significant difference at P ≤ 0.05.
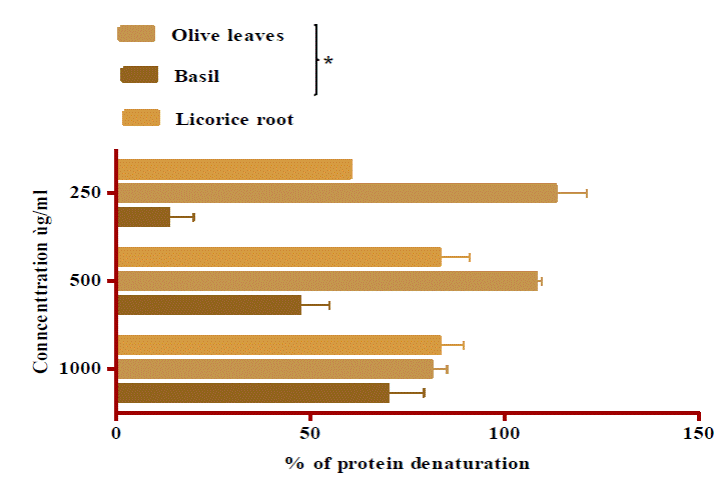
Figure 2: Inhibition of protein denaturation by the
extracts and BHT
Values are means ± SD of
three replicates (n=3)
3.4. Anti-oxidative assay
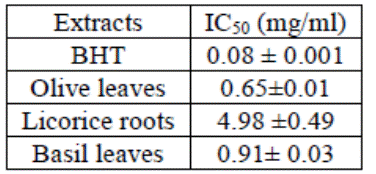
The
three extracts were less active than the BHT (0.08 mg/ml), OLE (0.65 mg/ml),
BLE (0.91 mg/ml) and LRE (4.98 mg/ml) (Table
3).
Table 3: IC50 values compared to BHT;
Values are means ± SD of three replicates (n=3)
3.3.4. Discussion
Water is almost universally the solvent used to
extract activity at home, dried plants can be ingested as teas (plants steeped in
hot water) or, rarely, tinctures (plants in alcoholic solutions) (Cowan, 1999). The yield obtained by
decoction of Basil was the highest compared to the other plants. This is due to
the fact that the part used of the plant influences its extraction yield; Basil
leaves are fine; those of the Olive are hard as far as for the Licorice root, which
influences the exchange surface between the solvent (water) and the used part of
the plant, despite the prolongation of the extraction time. According to (Dhanani et al., 2017), in conventional
extraction, heat is ransferred through convection and conduction from the surface,
the extractability of solvents depends mainly on the solubility of the compound
in the solvent, the mass transfer kinetics of the product and the strength of solute/matrix
interaction with corresponding limitations on heat and mass diffusion rate.
Decoction is the method of choice when working with tough and fibrous plants,
barks and roots and with plants that have water-soluble chemicals (Handa et al., 2008). Despite all, the
aqueous hot decoction remains effective, environmentally friendly in terms of solvent,
safe and not expensive.
3.2
Antibacterial assay
Olive leaves and Licorice roots extracts were active
on E. coli and S. aureus at the highest concentration with total
resistance of P. aeruginosa. The same, the OLE, tested by (Aliabadi
et al. 2012), showed
inhibitory effects at
50mg/ml on S. aureus(9mm) and E. coli(8.2mm). There was even inhibition of biofilm
formation of methicillin susceptible S.aureus and a
decrease in expression
and production of exotoxins
(hemolysins) and enterotoxins
(Rohinishree and
Negi, 2016;Alhamd et al., 2015). Indeed, there is
compelling scientific evidence that Olive leaf polyphenols are bioactive (antiviral
and antimicrobial). The polyphenol content
in the olive leaf could range from 1.5 to 7.0 g/100g. Oleuropein and other secoiridoids are the
principal (De
Leonardis et al.,
2008).The
water-soluble compounds, such
as polysaccharides and polypeptides,
are commonly more effective as
inhibitors of pathogen adsorption
(Cowan, 1999).While BLE
was completely inactive
on the three strains
tested. In a similar study, (Hiltunen and Holm (1999) have found that
the aqueous extracts or infusions of O.
gratissimum showed no activity against Salmonella spp., Shigella sp.
S. aureus and E. coli.
This indicates that
the antibacterial principles of this species are not water soluble. By
testing several types of solvents, Bacon et
al., (2016) found that the extracts from the hot water extraction
method showed no inhibition compared with the ethanolic and methanolic extracts.
Furthermore, fractionation confirmed the relative hydrophobic nature of active compounds. Contrariwise,
antimicrobial activity of Basil has been found against such organisms as
B. cereus, S. aureus (10mm), E. coli (7.5 mm) (Udochukwu et al., 2015), because Basil contains antimicrobial
compounds, one of them being eugenol these differences in antimicrobial properties
of plant extract is attributable to the age of
the plant used, the freshness of plant materials, physical factors
(temperature, light water), contamination by field microbes,
adulteration and substitution of plants, incorrect preparation and dosage (OkigboandMmeka, 2008). It should be noted that the recorded
activities are all bacteriostatic.
3.3.
Anti-inflammatory assay
Denaturation of proteins is a well-documented cause
of inflammation because non-steroidal anti-inflammatory drugs (NSAIDs) are
reported to possess prevention of the denaturation of proteins, which act as auto-antigens
and leads to auto-immune diseases or arthritis diseases (Karthik et al., 2013; Chatterjeeet al., 2012). In this survey, the maximum percentage of inhibition
was observed from OLE. Phenolic compounds, in
Olive leaves, including flavones, flavonols rutin), flavan-3-ols (catechin), substituted
phenols (tyrosol, vanillin and caffeic acid) and oleuropein can be responsible of
its activity (Abaza et al, 2015). In a close case, the aqueous suspension of Ocimum
sanctumleaves inhibited acuteas
well as chronic inflammation in rats in a way dose dependent
effect (Hiltunen and Holm, 1999). Study
of Ocimum basilicumcrude methanolic extracts
exhibited anti-inflammatory
activity as evidenced
by the inhibition
of the key
pro-inflammatory cytokines and mediators (Marwat et al., 2011).
The compounds of Licorice root, the glycyrrhizin, glycyrrhetinic acid
and glabridin were
confirmed to have
anti-inflammatory properties (Ghannadet
al., 2014; Tian et al.,
2008). The anti-inflammatory
mechanism of Licorice implies the exhibition of a teroid like anti-inflammatory
activity, similar to
the action of
hydrocortisone; this is
due in part,
to inhibition of phospholipase A2
activity, inhibition of
cyclooxygenase activity and
prostaglandin formation and inhibition of platelet aggregation (Zadeh et al., 2013).
3.4.
Anti-oxidative assay
The three extracts were less active than the
BHT. Despite this, the IC50values remain good by comparing them to other studies;
OLE from Turkey 59μg/ml (Orak et al., 2012),
from Iran 121.05 μg/ml (Rafiee et al., 2012),BLE from Egypt 53 mg/ml and
LRE from
Egypt 81% at 100 μg/ ml (Lawal et
al., 2016). Actually, there are many substances responsible
for the anti-oxidative effect such as β-carotene, tocopherol, eugenol, rosmarinic
and caffeic acids and linaloolin Basil (Hiltunen and Holm, 1999; Gbadegesin and Odunola, 2010). In Licorice, glabridin has
been reported to affect protection of mitochondrial functions from oxidative stresses
(Tian et
al., 2008). Experiments revealed that water extracts have
anti-oxidative effects which are concentration-dependent (Fig. 3) which
corroborates the results found by (Marwat et
al. 2011). According to (Cowan 1999), most active components are not
water soluble, his may be due to ability of organic solvents to extract the maximum
of bioactive compounds.

Figure 3: DPPH scavenging effect of the extract and this of BHT
Values are means ± SD of three replicates (n=3),
4.
Conclusions
Or study supports the use of active in treating
infections and inflammation which could be
further evaluated by other methods. However, further studies, including
the analysis of the extracts may result in the development of potent bioactive
agents with the minimum of toxicity. To confirm the use and to compare the results
found with previous works, it will first be necessary to standardize the
conditions of cultivation of the plants as well as the techniques of
extraction, the conditions of picking, packing, drying and storage conditions
in addition to the sale by herbalists which should be controlled by the local
authorities.
5.
Conflict
of interest statement
We certify that there is no conflict of interest with
any financial organization in the subject matter or materials discussed in this
manuscript.
6.
Authors’
biography
No Biography
7.
References
[1]
Abaza L. Taamalli
A. Nsir H
and Zarrouk M.
Olive Tree (Olea
europeaeL.) Leaves:
Importance and Advances
in the Analysis
of Phenolic Compounds.
Antioxidants, 2015. 4:682-698.doi:
10.3390/antiox4040682.PMID:26783953. PMCID: PMC4712944.
[2]
Alhamd AKJ. Improvement extraction of crude compounds
from iraqi olive leaves by appling water-base
and alcoholic-base extraction
and their biological
application. 2015. WorldJ Pharm
Sci, 4; 1:183-200. doi:10.3390/antiox6020034. PMCID:PMC5488014. PMID:28513570.
[3]
Aliabadi
MA. Darsanaki RK.
Rokhi ML. Nourbakhsh
M. and Raeisi
G. Antimicrobial activity of
olive leaf aqueous
extract. Ann Biol Res,
2012. 3(8):4189-4191. Available online at: www.scholarsresearchlibrary.com.
[4]
Bacon K. Boyer
R. Denbow C.
O’Keefe S. Neilson
A. and Williams
R. Evaluation of different
solvents to extract
antibacterial compounds from
jalapeno peppers. 2016.
Food Sci Nutr, 1-7. doi:10.1002/fsn3.423.
PMCID:PMC5448369. PMID:28572934.
[5]
Bouzabata A. Les
médicaments à base
de plantes en
Algérie: réglementation et enregistrement. 2016. Rev Phytother. 1-8. DOI
10.1007/s10298-016-1089-5.
[6]
Chatterjee P.Chandra S.Dey P. andBhattacharyaS.
Evaluation of anti-inflammatory effects of
green tea and
black tea: A
comparativein vitrostudy.J Adv
Pharm Technol Res, 2012. 3(2): 136-138.
doi:10.4103/2231-4040.97298. PMCID:PMC3401676PMID:22837963
[7]
Cowan MM. 1999. Plant product as antimicrobial agents.
Clin. Microbiol. Rev, 12 (4), 564-582. DOI:10.1128/CMR.12.4.564.PMID:
10515903 PMCID: PMC88925
[8]
De LeonardisA. AretiniA. AlfanoG.
and GiancarloR. Isolation of
a hydroxytyrosol-rich extract
from olive leaves (Olea EuropaeaL.) and evaluation of its antioxidant
properties and bioactivity.2008.EUR FOOD
RES TECHNOL.226(4):653-659. DOI:10.1007/s00217-007-0574-3.
[9]
DhananiT.ShahS. GajbhiyeNA.andKumarS. Effect
of extraction methods
on yield, phytochemical
constituents and antioxidant activity ofWithania somnifera.ARABJCHEM, 2017.
10(1): S1193–S1199. doi.org/10.1016/j.arabjc.2013.02.015.
[10] Gbadegesin MA. and
Odunola OA. Aqueous and ethanolic leaf extracts of Ocimum basilicum(sweet basil)
protect against sodium
arsenite-induced hepatotoxicity in
Wistar rats. 2010. Nig. J. Physiol. Sci, 25: 29-36. PMID:
22314900.
[11] GhannadMS.MohammadiA.
SafiallahyS.FaradmalJ.AziziM.andAhmadvandZ.
The Effect of Aqueous
Extract ofGlycyrrhiza glabraonHerpes Simplex
Virus1. Jundishapur J Microbiol,
2014. 7(7): e11616. doi:10.5812/jjm.11616. PMCID:PMC4216581.
PMID:25368801.
[12] Karthik K.
Kumar BR. Priya
and V. KumarS.
Evaluation of anti-inflammatory activity
of Canthium parviflorumby in-vitro
method. Indian Journal of Research in
Pharmacy and Biotechnology,2013.
729-730.
[13] Orak HO.
Isbilir SS. and
Yagar H. Determination
of antioxidant properties
of lyophilized olive water
extracts obtained from
21 different cultivars. Food Sci
Biotechnol. 2012. 1065-1074. DOI 10.1007/s10068-012-0138-6.
[14] Lawal B. Shittu OK.
Oibiokpa FI. Berinyuy EB. and Mohammed H. African natural products with
potential antioxidants and hepatoprotectives properties: a review. Clinical
Phytoscience, 2016. 2; (23):1-66. DOI:10.1186/s40816-016-0037-0.
[15] Zadeh JB.
Kor ZM. And
Goftar MK. Licorice(Glycyrrhiza glabra Linn)
As a Valuable Medicinal Plant. 2013. IJABBR, 1(10): 1281-1288.
[16] Singh G. Marimuthu
P. De Heluani CS. and Catalan CAN. Antioxidant and biocidal activities of Carum
nigrum(seed) essential oil, oleoresin, and their selected components. J Agric
Food Chem, 2006. 54: 174-181. DOI: 10.1021/jf0518610. PMID:16390196.
[17] Rebbas K. Bounar R.
Gharzouli R. Ramdani M. Djellouli Y. and AlatouD. Plantes d’intérêt médicinal
et écologique dans la région d’Ouanougha (M’Sila, Algérie). Rev Phytother, 2012. 10:131-142. DOI:
10.1007/s10298-012-0701-6.
[18] Marwat SK.
Rehman FU. And
Khan MS. Ghulam
S. Anwar N.
Mustafa G. et
al. Phytochemical constituentsand pharmacological activities
of sweet basil Ocimum
basilicumL. (Lamiaceae). Asian J. Chem,2011. 23(9): 3773-3782.
[19] Rohinishree YS.
and Negi PS.
Effect of extract
on cell viability,
biofilmformation and exotoxin
production by Staphylococcus aureus. J Food Sci Technol, 2016. 53(2):1092-1100.
DOI:10.1007/s13197-015-2131-6. PMID:27162389b PMCID: PMC4837708.
[20] Tian M. Yan H.and
Row KH. Extraction of glycyrrhizic acid and glabridin from licorice. Int J Mol
Sci, 2008. 9(4):571-7. PMCID:PMC2635700. PMID:19325770.
[21] Udochukwu U.
Omeje FI. Uloma IS.
And Oseiwe FD. Phytochemical
analysis of Vernonia amygdalinaand Ocimum
gratissimumextracts and their
antibacterial activity on
some drug resistant bacteria.
2015. Am. J. Res.3(5):225-235.
[22] Okigbo RN.
andMmekaEC. Antimicrobial effects
of three tropical
plant extracts onStaphylococcus
Aureus, Escherichia Coli and Candida Albicans. Afr J Tradit Complement Altern
Med, 2008. 5(3). PMID: 20161941. PMCID: PMC2816559. DOI:
10.4314/ajtcam.v5i3.31277.
[23] Rafiee Z.
Jafari SM. Alami
M. and Khomeiri
M. Antioxidant effect
of microwave-assisted extracts of
Olive leaves on Sunflower oil. J. Agr. Sci. Tech, 2012. 14: 1497-1509.
[24] [24]Reshma. Arun
KP. and Brindha
P. In
vitroanti-inflammatory,
antioxidant and
nephroprotective studies on leaves of
aegle Marmelos and Ocimum sanctum. Asian J Pharm Clin Res, 2014. 7(4):
121-129.
[25] Hammiche V.Merad
R. and Azzouz
M.Plantestoxiquesàusagemédicinaldupourtourméditerranéen,2013Springer-Verlag
France, Paris.
[26] HandaSS. Singh SP.
Longo KG. and Rakesh DD. Extraction Technologies for Medicinal and Aromatic
Plants. International Centre for Science and High Technology,
2008. Italy
[27] Rahal K. Standardisation de L’antibiogramme en Médecine Humaine à l’Echelle
Nationale selon les recommandations de l’OMS, fourth ed. Ministère de la Santé,
de la Population et de la Réforme Hospitalière, 2005. Algiers.
[28] LimTK.Edible
medicinal and non-medicinal plants. 2016. Springer.Vol 10
[29] Hiltunen R.
and Y Holm.
Basil The Genus Ocimum.
Tenth volume, harwood
academic publishers, 1999.
Amsterdam.