The Axis “Human Papillomavirus - Anal Squamous
Cell Carcinoma”: A Review
Type of article: Review.
Ana Carolina Borges Monteiro1, Reinaldo
Padilha França1, Valeria Tananska2, Abdeldjalil Khelassi3, Yuzo Iano1,
and Rangel Arthur4
1. State University of Campinas (UNICAMP), Brazil.
2. Medical University of Plovdiv, Plovdiv, Bulgaria.
3. University of Tlemcen, Algeria.
4. Faculty of Technology (FT) – State University of Campinas (UNICAMP),
Brazil.
Abstract
Background:
Anal Squamous Cell Carcinoma (ASCC) is an infrequent neoplasia that
represents 2% of the digestive tumors and it has a growing incidence.
Objective: This investigation
(i) studies the pathogenesis of an increasingly prevalent disease, (ii) its treatment
and prognosis along with (iii) a bibliographical review of the main
characteristics of the Human Papillomavirus (HPV) as well as its effects on humans.
Methods: A
literature review is performed, comprising articles up to 2019 and
cross-research manuscripts with the initial research.
Results: Several
studies demonstrate the HPV role as a significant risk factor to the
development of ASCC, as well as its higher incidence in HIV-positive individuals
and in those who engage in receptive anal intercourse. Future trends in
theragnostic using information technology are examined.
Conclusions:
ASCC is a neoplasm mostly associated with HPV. Many studies are needed
to improve the treatment as well as in the evaluation of the tumor characteristics.
Keywords: Human Papillomavirus, HPV, Anal Squamous Cell Carcinoma, ASCC, STD, Anal Canal Lesions, Anatomy, Histopathology, HIV.
Corresponding author: Ana Carolina Borges Monteiro, State University
of Campinas (UNICAMP), Brazil email: carol94monteiro@gmail.com
Screened by iThenticate..©2017-2019 KNOWLEDGE
KINGDOM PUBLISHING.
1. Introduction
Anal Squamous Cell Carcinoma (ASCC) is cancer on the rise. According to the Global
Cancer Observatory (GCO), there were well over 48,000 new cases in 2018
alone, with Asia, Europe and North America holding the top three positions for
incidence in both sexes [1]. ASCC impacts the mucosa, submucosa and
the muscularis of the anal canal. Prior detection frequently encountered patient
complaints include pain and prolonged anal bleeding. The diagnostic investigation involves
visual and histological anorectal examination, and, at a later stage - anoscopy
or rectoscopy. Deciding on a final diagnosis is slow to come as initial complaints
mirror those of external or internal hemorrhoids.
ASCC’s onset is a
person-specific process. A common denominator seems to be infection with the human
papillomavirus (HPV), present in 70 – 90% of diagnosed ASCC cases [2]. HPV is a
virus with tropism by differentiating tissues. Its pathogenesis is related to
the disorder of genes that inhibit cell apoptosis and cell suppression. This
fact favors its action and spread by an organism. Due to these characteristics,
HPV is associated with cervical, anus, head and neck cancer.
The current article explores
the HPV - ASCC connection in Section 2. While it reflects scientific research on the matter published between
2003 and 2019, the article also provides in-house specialist analysis. The
macro- and micro-anatomy of the anal canal are discussed in Section 3. Section
4 examines cancer precursor lesions. The ASCC is investigated in Section 5.
Current treatments, future trends, and conclusions appear, respectively, in
Sections 6, 7, and 8.
2. Human
Papillomavirus (HPV)
The study of human papillomavirus
(HPV)-related infections is relatively new. Its origins date back to the 1970s
when Prof. Harald zur Hausen, chairman of the Institute of Virology at the
University of Freiburg, Germany, chose HPV as the center-theme of his research.
Studying cervical cancer biopsies, in 1983, Prof. zur Hausen’s team was
successful in isolating the DNA of the high onco-risk HPV-16 variant, and in
1984 – that of HPV-18. For his significant contributions to science, Prof.
zur Hausen went on winning the 2008 Nobel Prize in Medicine. Based on his
findings, HPV-related research has since flourished [3, 4, 14].
HPV is a frequently
encountered virus. Statistics show that 2/3 of all sexually active people (irrespective
of involvement in vaginal or anal sex) have acquired it. During the first two
years post defloration, 40% of women fall victim to it. Condoms do not prevent
infection.
The virus has many
strains. Not all of them are oncogenic, and the immune system can suppress them
successfully. The non-oncogenic HPV infection often manifests visually as warts
or condyloma acuminatum. The oncogenic
strains of the virus can either stay dormant for years (embedded in anal
tissues [5] or circulating in the blood and lymphatic systems, thus reaching
other tissues and organs) or starts replicating immediately post-infection. In descriptive terms, HPV is a 72-capsomer,
non-enveloped virus, belonging to the Papovaviridae family. Its mean diameter is
55 nm. HPV is a recombinant retrovirus. Retroviruses transform the single-stranded RNA genome they
carry into a double-stranded DNA molecule that integrates into the genome of
dividing target cells. HPV’s genes encode 2 structural (L1 and L2) and 7
non-structural (E1, E2, E4, E5, E6, E7) proteins [15-21].
The described genes are organized in three
regions: an early region (E), a late region (L), and a regulatory region (URR).
The L1 and L2-encoding genes are responsible for the structure of the viral
capsid, as well as for proteins involved in viral replication and cell
transformation. When L1 is produced in a heterologous expression system, it can
self-assemble. The E1 and E2-encoding genes contribute to viral replication. The
E5, E6, and E7-encoding genes take care of proteins responsible for infected cells’
transformation.
Based on the histological target of their action,
HPV infections can be divided into two major types: cutaneous or mucosal [6].
Current scientific literature shows a
further differentiation into over 200 strains. Approximately 45 of them target
the anogenital tract. In terms of the potency of their oncogenic potential, HPV’s
strains can be classified as low-risk (types 6, 11, 42, 43 and 44) and high
risk (types 16, 18, 31, 33, 35, 39, 45, 46, 51, 52, 56, 58, 59 and 68) [6] [7].
HPV infection can ensue in many ways. The
virus can disseminate through direct contact with surface lesions on an
infected human body (e.g., oral cavity, skin), or contact with desquamated
cells or body fluid residue left on previously touched the inanimate surface
[22-30].
Vertical transmission during pregnancy and
delivery is observed on occasion.
The HPV infection is also the most
frequently encountered, undesirable result of unprotected sex involving anorectal
intercourse.
3. Macro- and Micro-Anatomy of the Anal Canal
The anal canal is not a part of the human
reproductive system. As such, it has no gender. It does not lead to an organ
supporting impregnation and the creation of life. The anal canal is the
terminal portion of the gastro-intestinal (GI) tract. Following rises in
intra-abdominal pressure, it serves for the expulsion of feces– by-products of
human food consumption and the recycling of heme. The passage is
mono-directional – from the anal canal, out of the human body [32-36].
Macro-anatomically speaking, the anal canal
is approximately 3 cm long. It extends from the lower margin of the rectum up to
the external margin of the anus.
In its upper half, the lumen of the anal canal
exhibits 8 – 10 vertical columns. Also known as anal or rectal columns (columns
of Morgnani), they are produced by the push-out of the mucosa by the
longitudinal outer muscle layer of the rectum. In their upper portion, the anal
columns are raised. Moving downwards, they gradually flatten out.
Adjacent anal columns are separated via furrows.
Below, the so-called anal sinuses are limited by the anal valves (Ball’s) -
transverse mucosal folds with a half-moon shape.
Collectively, the anal valves form a line known as the
anal pecten.
The space between the anal pecten and 8 mm to the external
margin of the anus is called the zona haemorrhoidalis. Here, the mucosa is
smooth.
The submucosa contains the haemorrhoidal
plexus. The soft, malleable nature of the haemorrhoidal plexus protects the
mucosa from mechanical harm of passing hard feces. It is also instrumental in
the tight closing of the anus.
The zona haemorrhoidalis terminates with
the pectinate line (Hilton’s white line).
Beyond the pectinate line, up to the external
margin of the anus, lies zona cutanea - the skin-covered portion of the anal
canal. The zona cutanea contains hair follicles, sebaceous and sweat glands.
Normal anal continence is maintained via muscles
of the pelvic floor - the levator ani and the sphincter ani internus
(involuntary) et externus (voluntary), as well as the aforementioned plexus haemorrhoidalis.
The upper half of the anal canal (above the anal pecten) is sensitive to
stretch, while the bottom half - to pain, touch, and temperature differences [8].
The viscerosensory experiences in the anal canal are explained through the
action of submucosa’s haemorrhoidal plexus, muscularis’ myenteric plexus (Auerbach’s)
and the mechanoreceptors Vater-Pacini corpuscles.
The normal histology of the anal canal
shows a smooth, top-down transition:
§ starting below the lower margin of the rectum with
-
the simple
columnar epithelium of the anal columns
§ passing below the anal pecten with
-
the non-keratinized
stratified squamous epithelium of the zona haemorrhoidalis (histologically
marked as the anal transition zone), and
§ ending below the pectinate line with
-
the keratinized
stratified squamous epithelium of anus’ epidermis.
4. Cancer Precursor Lesions
During
anorectal intercourse, the pronounced mismatch between the circumference of the
glans penis and the maximum stretching capability of the external and the
internal anal sphincters determines the rougher thrusting nature of the sexual
act. The outcome is the presence of mid-size and deep abrasions - traumatic
desquamation, often combined with longitudinal lesions and rugae (Figure 1).
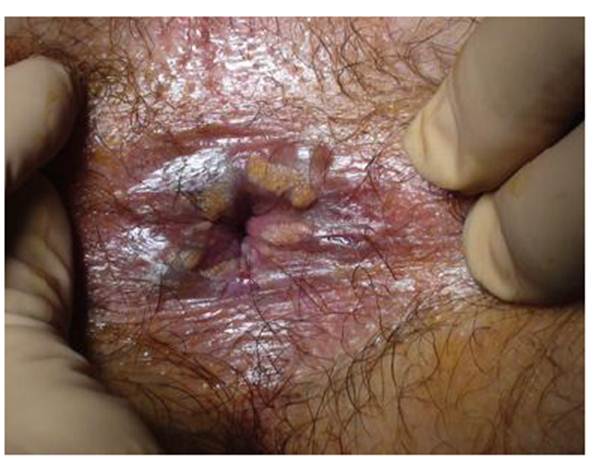
Figure 1. Example of external anal margin lesions, consistent with
histological changes due to previous anorectal intercourse (photo courtesy of
Société Nationale Française de Colo-Proctologie, [5])
The
abrasions expose both sex partners to fecal bacteria present in the anal canal.
The contact between
the by-products of fecal bacteria’s living cycle and the HPV may cause mutation
of the virus.
Abrasions
may also increase the risk for infection with parasites, other viruses (e.g. Herpes simplex virus,
HSV) [9], as well as sexually-transmitted
bacteria. Some notable examples of the latter are:
§ Campylobacter jejuni
§ Chlamydia trachomatis
§ Neisseria gonorrhoeae
§ Shigella
sonnei
§ Haemophilus
ducreyi
§ Calymmatobacterium
granulomatis
§ Treponema
pallidum [10]
For a
healthy receiving sex partner, the abrasions ensure HPV’s access not only to anus’
epidermis, but also to the deeper layers of the anal mucosa (lamina propria and
associated blood and lymph vessels), the submucosa, and muscularis. Abrasions
in the anus and the anal canal of an HPV-positive receiving partner lead to
leakage of the virus onto the glans penis’ mucosa, the prepuce, and the penis
body’s skin of a healthy thrusting sex partner.
The
integrity of the penis of the latter is already compromised, as a result of the
rough friction between the penile mucosa and skin on one side, and the anus’
skin and the mucosa of the anal canal on the other. Micro-hemorrhaging occurs. As
a result, the leaked HPV virus enters the healthy thrusting sex partner’s blood
and lymph circulation. A worse effect happens
when an HPV-positive thrusting sex partner causes abrasions onto the anal
tissues of a healthy receiving sex partner.
Due to the
rough nature of the anorectal intercourse, the penis of the infected thrusting
partner causes abrasions, while pushing the virus deep inside them. Observed is
a simultaneous “plow and seed” action of sorts. Desquamated epidermal cells
from the skin of the body of the HPV-positive penis remain in the anal canal.
With deeper penetration, they reach the rectum. The contact of the HPV virus
and foreign skin cells with the abrasions triggers an immune system defense response.
Other known symptoms of anorectal intercourse with HPV infection may cause are pruritis
ani, pain, rectal bleeding, and mucus or fecal
discharge [9].
5. Anal Squamous Cell Carcinoma (ASCC)
Once HPV
infects the nuclei of host cells, the virus follows two routes – it either
activates and replicates, or, most often than not, stays dormant for years
prior to the onset of detectable symptoms [6]. This period of latency is known
as a “window period” and its duration is affected by a list of factors:
§ engagement in sexual intercourse since an early age
§ multiple sexual partners
§ high number of non-surgically assisted births
§ young chronological age
§ smoking
§ low socioeconomic status
§ prolonged use of oral contraceptives
§ fistulas
§ nutritional factors [58, 59]
§ Human Immunodeficiency Virus (HIV) infection
§ other infections caused by agents throughout sex-related activities
(e.g., Chlamydia trachomatis, Herpes simplex virus) [11].
As it was already
mentioned, in 70 - 90% of anal HPV-positive cases, the end of the “window
period” marks the rise of ASCC-related patho-histological changes.
ASCC is a collective term
used to describe three sub-types of squamous cell carcinoma - large cell
keratinzing, large cell non-keratinizing and basaloid. The three sub-types do
not differ significantly in their prognostic features. Data from the US
National Cancer Database, processed by the American Joint Committee on Cancer
(AJCC), shows that more advanced ASCC is inversely proportionate to patients’
survivability rates.
ASCCs have localized expression
with or without associated regional lymph node activation. In the case of the
latter, it has been noted that tumors found above the
pectinate line spread primarily to the anorectal, perirectal, and internal
iliac lymph nodes. Tumors below the pectinate line impact mostly the
superficial inguinal lymph nodes. ASCC can metastasize to any distal
organ. Yet, the liver and the lungs seem to be the most frequently impacted.
Secondary spread to the abdominal cavity is not unheard of [12]. Some examples of ASCCs
are as follows:
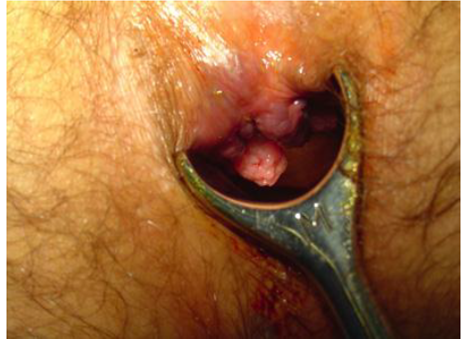
Figure 2. ASCC with out-growths restricted to the anal canal (photo
courtesy of Société Nationale Française de Colo-Proctologie, [5])
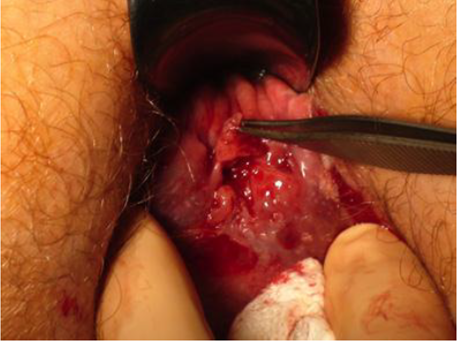
Figure
3. Rugae or fissure-incorporating ASCC (photo courtesy of Société Nationale
Française de Colo-Proctologie, [5])
ASCC diagnosis starts
with a visual examination of the affected area. In the absence of epithelial
out-growths, an anal Pap smear test is administered. An anal
swab or endocervical brush is introduced in a full circular motion into the
anal canal. The collected cells and mucus are then histo-pathologically
processed [13].
The anal Pap smear
test is usually accompanied by more invasive diagnostic methods - an anoscopy,
and on occasion, rectoscopy. Anoscopy allows better visual evaluation of the
number, depth, distribution, and position of HPV-infected lesions, and rugae. Based
on the experience of the proctologist performing the anoscopy, the latter is
instrumental in recognition of areas of morphological urgency, i.e., in need of
performing an immediate biopsy. Such need is also deemed proper in the
presence of suspicious mucosal out-growths like the ones visualized in Figures
2 and 3. Then, the discovered ASCCs are
classified according to the anatomically-driven TNM Classification system, devised
by the Swiss-based Union for International Cancer
Control (UICC). Each patient’s condition is coded with the aid of
three letters:
§
T -
indicates the primary tumor site
§
N -
notes any regional lymph node involvement
§
M - draws
attention to the presence of close or distant metastases.
To denote the specific stage
of ASCC’s development, TNM’s classification system employs Roman numerals (i.e., stage I, II, III, and IV cancer).
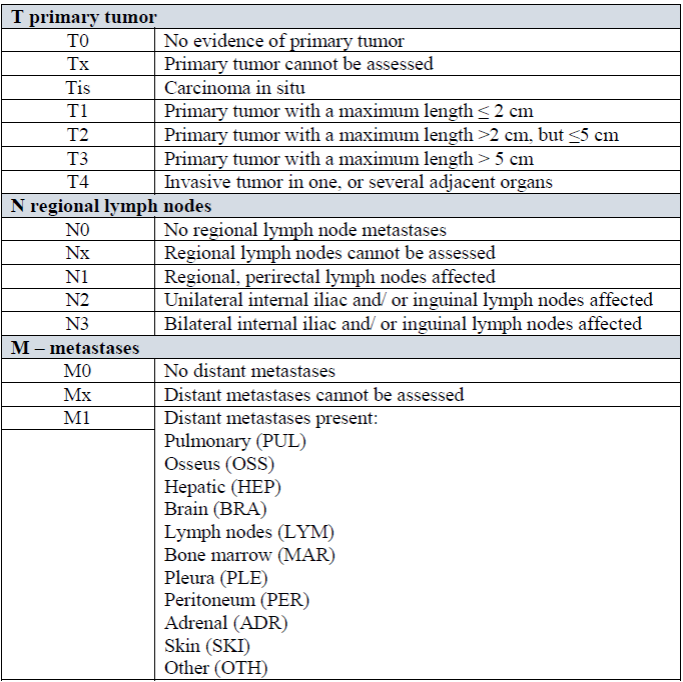
Table
1. TNM classification of histopathological findings in the anal canal [36].
ASCCs’ pre-medicatory treatment
potential is determined clinically (cTNM), following information collected from
the visual examination, lab results from the anal Pap smear test, and
histopathological evaluation of collected biopsy material. Due to the high
immunogenicity of HPV-positive ASCC with lymph node activation. However, the
majority of tumors are surgically excised.
Data obtained from the
procedure and the subsequent histopathological examination of the excised mass
allows a post-surgical pathologic classification (pTNM). The pTNM is then used
to establish strategies for post-surgical adjuvant therapy and follow up [35]. In
an attempt to predict patient survivability, the American Joint Committee on
Cancer improved on the TNM classification:
Table 2. The hybrid AJCC- TNM classification of
histopathological findings in the anal canal [12].

For tumors that cannot be removed, the
international standard of medical care recommends chemoradiotherapy (CRT) using
5-fluorouracil and mitomycin C. It has been noted that about 30% of patients do
not respond positively to the treatment [2].
6. Future Research
Future scientific work on
the HPV - ASCC axis must target a timely, correct diagnosis of anal squamous
cell carcinoma.
They should start with
deep sequencing of fecal bacteria, matched with the observation on bacteria’s interaction
with anal canal lesions and the HPV, alike.
Additional efforts should
also be paid to the global distribution of anal HPV strains, once they have
reached the blood and the lymphatic systems. This avenue should reflect research
on the mutation capabilities of the virus in radically different tissue
environments to the original one.
As most contemporary external
imaging methods have not been particularly instrumental to the proper diagnosis
of ASCC (and anoscopy/ rectoscopy are traumatic for the anal canal and the
patient), a new human operator or Artificial Intelligence (AI) driven, light-, camera-
and biopsy pince-fitted micro-robotic entities for anal entry are needed. Some discussions follow.
A) Image
Analysis:
Computer image analysis can help to
evaluate the alterations in the cells to discern between benign and malignant
lesions where the samples can be obtained through biopsies and diagnosed as
ASCC [38, 39].
The ever-growing availability of
digital histopathological images augmented the demand for their automatic analysis,
e.g., computer-aided diagnosis via machine learning. However, digital pathology
and related tasks must consider some issues. Novel digital pathological
techniques within image analysis can arise from the more intensive use of
computational intelligence to address some particular and unsolved problems and
recommend possible solutions [40-46, 61].
B) Multimodal
Imaging
There are several types
of image processing equipment. Each kind comprises an imaging modality.
Positron Emission
Tomography (PET) and Computed Tomography (CT) scans are varieties of medical
examination equipment that can be currently used in the theragnostics (that is,
by selecting the best biopsy site, assessing the treatment response, seeking
other related tumors, searching suspect tumor recurrence with markers, and
radiation treatment planning) of cancers of several sites [46]. Since many
tumors seek fluoro-D-glucose (FDG), a high FDG uptake is customarily linked
with a high manifestation of glucose transporters. However, an increased FDG uptake does not necessarily
indicate neoplasms because inflammatory processes may also show increased
uptake (such as abscesses, fungal infections, tuberculosis, diverse types of
inflammations, and inflammations related to radiation usage among others that
cause false-positive results) [46, 47].
The Tissue Microarray
(TMA) is a high-throughput technology employed in oncology to investigate
molecular markers. It allows the rapid evaluation of biomarkers in thousands of
tumor samples, using commonly available laboratory assays such as
immunohistochemistry and in-situ hybridization. TMA has proven to be valuable
to study tumor biology, help to develop diagnostic tests and explore
oncological biomarkers. Up to now, TMA has a significant impact on clinical
oncology, and it promises more potential applications [48, 49].
Multispectral Imaging
(MSI) and Hyperspectral Imaging (HSI) comprise new modalities for biomedical
applications initially developed for remote sensing [50]. They can extend
vision to infrared in addition to near-infrared wavelength regions of the
electromagnetic spectrum. One can use a Multispectral Image (MSI) or Hyperspectral
Image (HSI) that, in combination with another immunohistochemical approach, can
pinpoint and quantify immune cells in diagnostic tissue samples. The resulting
images can be related to traditional visual evaluation of immune cells from an
extensively annotated TMA to correlate immune cell counts from adjacent tissue
sections with knowledge about the immune cells’ distance mapping and the immune
signatures associated with clinical parameters [51].
Whole Slide Imaging (WSI)
or scanning for TMA core annotation and region selection for MSI/HSI can be
done. A pathologist can visually examine the scanned image once the initial
image analysis is ready. Additionally, regions/samples with staining artifacts
and with large necrotic areas can be left out. Image processing comprises the
training session and image analysis session. The training session can include
manual annotation of three region types: tumor, stroma, and blank areas. Then,
a machine-learning-based algorithm can execute the tissue segmentation based on
the nuclear 4′,6-diamidino-2-phenylindole (DAPI) staining, for instance [39-45,
52].
Despite the overall
reliability of the data produced by MSI and HSI, some limitations should be
mentioned. There is some degree of crosstalk between a couple of the
fluorophores with overlapping emission spectra. MSI unmixing of fluorescent signals
is sensitive to deviations in the signal profile that may be slightly changed when
staining becomes very intense. Future studies can solve this problem by ensuring
that no cells are stained above a certain threshold. Studies must be validated
using benchmark imaging datasets before utilization with more specific diagnostic
tissue samples. Image analysis enables immune cell classification, along with
the creation of in situ maps containing the spatial distributions of cells. The
specific prognostic effects of different immune cell constellations can
emphasize the use of this diagnosing strategy and, in the future, it may be
part of the immune status routine characterization of cancer patients.
C) Robotics
The prevention, early
discovery, rapid diagnosis and timely management of cancer are crucial.
Information Technology (IT) can expand the patient survival rate and increase
the satisfaction of patients, caregivers, and healthcare providers as far as
cancer goes [64]. Robots are utilized in different healthcare areas and their
applications in surgery have arisen to the cancer treatment realm. IT devices
can boost dexterity, efficient motion scaling skills while providing high-quality
3D computer vision for surgeons with reduced loss of blood, a noteworthy decline
in narcotic usage, and low hospital stay period for patients. Nevertheless,
many challenges persist, such as the absence of surgical community support, high
costs, availability of different sizes, and lack of tactile/haptic feedback. Surgeons
also need more evidence and proper support from physicians [57, 60, 62, 63].
Microbeads, microgels,
and other nanodevices can be assembled within magnetic fields [53-56] to
provide a cost-effective theragnostic without potentially toxic interactions. External
magnetic fields can control these micro-robots and nanorobots. Their motion can
be actuated accurately to bring together 2-D and 3-D hydrogels that encapsulate
several types of cells. These methodologies deliver new ways to handle 3D engineering
structures and offer extensive potential usages in
regenerative medicine, experimental biology, and drug screening, among other
scenarios.
Research with robotic surgery for cancer patients will
continue because some patients are unable to undergo manually guided surgery or
other invasive, high-risk procedures [56, 57].
Robotics will also help advances in 3D and 4D imaging
with different types of cameras, augmented reality options, image processing
techniques, and 3D printing [65-68].
Advances in databases
will also impact ASCC theragnostic [69].
7. Conclusion
ASCC is a neoplasia
mostly associated with HPV. Future studies will improve its theragnostic. This
research is organized in a three-fold fashion: (i) studies about the pathogenesis of the disease,
(ii) its theragnostic along with (iii) a bibliographical review of the central
HPV characteristics, and the way it
affects people.
Studies such as this can
be seen as crucial elements for understanding and preventing this Sexually
Transmitted Disease (STD), which has its highest incidence rates in
underdeveloped and developing countries, where health and education policies
are often scarce or nonexistent.
Chemoradiotherapy is the
treatment of choice, with abdominoperineal resection kept for the cases of
failed treatment or recurrence. Evidence progresses to adjust the treatment to
patients individually, considering each person prognostic elements and
biological tumor features. Hence, among the prevention measures, one can cite
are the screening and vaccination programs of male individuals.
IT will bring in several
improvements to ASCC theragnostic.
8. Conflict of interest statement
We certify that there is no
conflict of interest with any financial organization in the subject matter or
materials discussed in this manuscript.
9. Authors’ Biography
Ana Carolina
Borges Monteiro: B.Sc. in Biomedicine from Centro
Universitario Amparense - UNIFIA, Brazil (2015). Currently, she pursues a D.Sc.
degreee from the Department of Communications (DECOM), Faculty of Electrical
and Computer Engineering (FEEC) at the State University of Campinas (UNICAMP),
Brazil, and she is a researcher at the Laboratory of Visual Communications (LCV). She is also
the Registration Chair and a reviewer
for the Brazilian Symposium on Technology (BTSym) and has expertise in the
areas of clinical analysis, histology, biomedical engineering, image processing
and the medical internet of things. She operates several types of electronic
medical equipment, has some knowledge on microscopy and some programming
experience in MATLAB. She has performed work, research experiments/projects,
and internship in municipal hospitals.
Dr Abdeldjalil Khelassi: is an Associate Professor at Tlemcen University,
Algeria. He obtained his Doctor in Science (2013), Magister (2008) and Engineer
(2004) in Computer Sciences from the Department of Computer Science at Tlemcen
University. His research interest includes cognitive systems, knowledge-based
systems, case-based reasoning, distributed reasoning, fuzzy sets theory and
health science. He is the editor manager of Medical Technologies Journal and
associate editor at Electronic Physician Journal.
Yuzo Iano: B.Sc., M.Sc., and Ph.D. degrees (1986) in Electrical
Eng. at UNICAMP, Brazil. He has been working in the technological production
field, with 1 patent granted, 8 filed patent applications and 36 projects
completed with research and development agencies. He has supervised 29 doctoral
theses, 49 master’s dissertations, 74 undergraduate and 48 scientific
initiation works. He has participated in more than 100 master’s examination
boards, 50 doctoral degrees, author of 2 books and more than 250 published
articles. He is currently a professor at UNICAMP, Editor-in-Chief the SET
International Journal of Broadcast
Engineering and General Chair of the Brazilian Symposium on Technology (BTSym).
He has experience in Electrical Engineering, with knowledge in
Telecommunications, Electronics and Information Technology, mainly in the field
of audio-visual communications and multimedia.
Rangel Arthur
Reinaldo Padilha França
Valeria Tananska
10. References
[1] Global Cancer Observatory (GCO). Cancer Fact
Sheets. <https://gco.iarc.fr/today/data/factsheets/cancers/10-Anus-fact-sheet.pdf>
access 08.11.2019.
[2] Martin, D.,
Balermpas, P., Winkelmann, R, Rödel, F., Rödel, C, Fokas, E. (2018). Anal
squamous cell carcinoma - State of the art management and future perspectives.
Cancer Treatment Reviews. 65, 11-21. https://doi.org/10.1016/j.ctrv.2018.02.001 PMid:29494827
[3] Bosman, F.T., Carneiro, F., Hruban, R.H., et al.
(2010). WHO classification of tumours of the digestive system, 4th edition.
Lyon: International Agency for Research on Cancer. (3), 184-93.
[4] Harald zur Hausen. Nobel Prize Award'
Biographicals.
<www.nobelprize.org/prizes/medicine/2008/hausen/biographical/> access
09.11.2019.
[5]
Le cancer de l'anus. Société Nationale Française de Colo-Proctologie. <
www.snfcp.org/informations-maladies/cancer/cancer-de-lanus-2014/> access
09.11.2019.
[6] Hellner, K., Munger, K. (2011). Human
papillomaviruses as therapeutic targets in human cancer. Journal of Clinical
Oncology, 29(13), 1785-94. https://doi.org/10.1200/JCO.2010.28.2186 PMid:21220591 PMCid:PMC3675666
[7] Doorbar J., Egawa, N., Griffin, H., Kranjec, C.,
& Murakami, I. (2015). Human papillomavirus molecular biology and disease
association. Reviews in medical virology, 25, 2-23. https://doi.org/10.1002/rmv.1822 PMid:25752814 PMCid:PMC5024016
[8] Maxwell
J.H., Khan S., Ferris R.L. (2015). The molecular biology of hpv-related head
and neck cancer. In: Fakhry C., D'Souza G. (eds) HPV and Head and Neck Cancers.
Head and Neck Cancer Clinics. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2413-6_4
[9] Snell, R.
S. (2012). Clinical anatomy by regions, 9th ed. Wolters Kluwer-Lippincott
Williams & Wilkins.
[10] Beck, D.
(2011). Sexually transmitted diseases. ASCRS Textbook of Colon and Rectal
Surgery, 2nd ed. New York: Springer : 295-307.https://doi.org/10.1007/978-1-4419-1584-9_17
[11] Whitlow,
C.B. (2004). Bacterial Sexually Transmitted Diseases. Clinics in Colon and
Rectal Surgery. 17(4): 209-214 https://doi.org/10.1055/s-2004-836940
[12] AJCC 7th Ed Cancer Staging Manual, 7th ed., ch.15
Anus., 181 - 6 (2015). <
https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%207th%20Ed%20Cancer%20Staging%20Manual.pdf>
accessed 9.11.2019.
[13] Okami, K. (2016). A new risk factor for head and
neck squamous cell carcinoma: human papillomavirus. International Journal of
Clinical Oncology, 21(5), 817. https://doi.org/10.1007/s10147-016-1012-y PMid:27368335
[14] Mammas, I.
N., & Spandidos, D. A. (2017). Paediatric Virology as a new educational
initiative: An interview with Nobelist Professor of Virology Harald zur Hausen.
Experimental and Therapeutic Medicine. 14(4), 3329-3331. https://doi.org/10.3892/etm.2017.5006 PMid:29042913 PMCid:PMC5639320
[15]
Jesus, S. P. D., et al. (2018). A high prevalence of human
papillomavirus 16 and 18 co-infections in cervical biopsies from southern
Brazil. Braz. J. Microbiology, 49, 220-223. https://doi.org/10.1016/j.bjm.2018.04.003 PMid:29720351 PMCid:PMC6328718
[16] Ribeiro, A. A., Costa, M. C., Alves, R. R. F.,
Villa, L. L., Saddi, V. A., dos Santos Carneiro, M. A., & Rabelo-Santos, S.
H. (2015). HPV infection and cervical neoplasia:
Associated risk factors. Infectious Agents and Cancer, 10(1), 16. https://doi.org/10.1186/s13027-015-0011-3 PMid:26244052 PMCid:PMC4524198
[17] Cseke, L. J., Kirakosyan, A., Kaufman, P.
B., & Westfall, M. V. (2016). Handbook of
molecular and cellular methods in biology and medicine. CRC Press. https://doi.org/10.1201/b11351
[18] Erkekoglu,
P. (2019). Oncogenes and Carcinogenesis. https://doi.org/10.5772/intechopen.74727
[19]
Abreu, M. N. S., et al. (2018).
Conhecimento e percepção sobre o HPV na população com mais de 18 anos da cidade
de Ipatinga, MG, Brasil. Ciência & Saúde Coletiva, 23, 849-860. https://doi.org/10.1590/1413-81232018233.00102016 PMid:29538565
[20] Allen, D. C.,
Cameron, R. I. (Eds.) (2017). Histopathology specimens: clinical,
pathological and laboratory aspects. Springer.
[21] Minsky, B.
D., Guillem, J. G. (2016). Neoplasms of the anus. Holland‐Frei Cancer
Medicine, 1-12.
[22] Júnior,
J.C.M.S. (2007). Câncer Ano- retocólico - Aspectos atuais: I - Câncer Anal. Rev.
Bras. Coloproct;27(2): 2109-223. https://doi.org/10.1590/S0101-98802007000200016
[23]
Nadal, S.R; et al. (2009). Quanto
a escova deve ser introduzida no canal anal para avaliação citológica mais
eficaz? Rev Assoc Med Bras; 55(6): 749-51. https://doi.org/10.1590/S0104-42302009000600022 PMid:20191232
[24] Duarte, B. F., da Silva, M. A. B., Germano, S.,
& Leonart, M. S. S. (2016). Anal cancer
diagnosis in patients with human papilomavírus (HPV) and human immunodeficiency
virus (HIV) coinfection. Rev Inst Adolfo
Lutz. 75, 1710.
[25] Monteiro, A. C. B., da Cruz Pires, D. V. D. (2015). Characterization of the risk factors for anus cancer
and its relationship with Human Papillomaviruses. Rev. Saude em Foco. https://doi.org/10.17648/unifia-saude-foco-ed-8-vol-1-032
[26] Chaves, E. B. M., Capp, E., Corleta, H. V. E.,
Folgierini, H. J. (2011). A citologia na prevenção do câncer anal. Femina: Rio
de Janeiro. 39(11), p. 532-537.
[27] Cuevas, M. (2019). Virus del papiloma humano y salud
femenina. Ediciones i.
[28] Magalhães, M.N., & Barbosa, L.E.: Anal canal
squamous carcinoma. J. Coloproctol. 37(1), 72-79, (2017). Doi:
10.1016/j.jcol.2016.08.003
[29] Cutrim, P. T.: Papilomavírus humano (hpv) e sua
associação entre as lesões cervical e anal em mulheres (2017).
[30] Darragh, T. M., Palefsky, J. M. (2015). Anal
cytology. In The Bethesda System for Reporting Cervical Cytology (pp. 263-285).
Springer, Cham. https://doi.org/10.1007/978-3-319-11074-5_8
[31] Bernardy,
J. P., Bierhals, N. D., Possuelo, L. G., & Renner, J. D. P. (2018). Padronização da PCR em tempo real para a genotipagem de
HPV 6-11, HPV 16 e HPV 18 utilizando controle interno. Revista Jovens Pesquisadores, 8(1), 37-48.
https://doi.org/10.17058/rjp.v8i1.12090
[32] Clifford,
G. M., et al. (2016). Comparison of two widely-used HPV detection and
genotyping methods: GP5+/6+ PCR followed by reverse line blot hybridization and
multiplex type-specific E7 PCR. Journal of Clinical Microbiology, JCM-0061. https://doi.org/10.1128/JCM.00618-16 PMid:27225411 PMCid:PMC4963525
[33] Wang, X., et al. (2014). MicroRNAs are biomarkers
of oncogenic human papillomavirus infections. Proc. National Academy of
Sciences of the United States of America, 111(11), 4262-4267. https://doi.org/10.1073/pnas.1401430111 PMid:24591631 PMCid:PMC3964092
[34] Allison,
D. B., Olson, M. T., Maleki, Z., & Ali, S. Z. (2016). Metastatic urinary
tract cancers in pap test: Cytomorphologic findings and differential diagnosis.
Diagn. Cytopathology, 44(12), 1078-1081. https://doi.org/10.1002/dc.23543 PMid:27434279
[35] Greene,
F.L. (2003).TNM staging for malignancies of the digestive tract: 2003 changes
and beyond. Seminars in Surgical Oncology. 21, 23 - 9. https://doi.org/10.1002/ssu.10018 PMid:12923913
[36] TNM classification system for cancer. UICC.
<www.uicc.org/resources/tnm> access 09.11.2019.
[37] Monteiro, A.C.B., Iano, Y., França, R.P., Arthur
R., Estrela, V.V. (2019). A comparative study between methodologies based on
the Hough transform and watershed transform on the blood cell count. In: Iano,
Y., Arthur, R., Saotome, O., Estrela, V. V., Loschi, H.J. (eds) Proc. 4th Braz.
Technology Symposium (BTSym'18). Smart Innovation, Systems and Technologies,
vol 140. Springer, Cham. doi: 10.1007/978-3-030-16053-1_7
[38] Gurcan,
M.N., Boucheron, L.E., Can, A., Madabhushi, A., Rajpoot, N.M., & Yener, B.
(2009). Histopathological image analysis: A review. IEEE Reviews in Biomedical
Engineering, 2, 147-171. https://doi.org/10.1109/RBME.2009.2034865
PMid:20671804 PMCid:PMC2910932
[39] Razmjooy, N., Estrela, V.V., Loschi, H.J.
(2019). A study on metaheuristic-based neural
networks for image segmentation purposes, in Q. A. Memon, S. A. Khoja (eds)
Data Science Theory, Analysis and Applications, Taylor and Francis.
https://doi.org/10.1201/9780429263798-2
[40] Komura,
D., & Ishikawa, S. (2018). Machine Learning Methods for Histopathological
Image Analysis. Computational and Structural Biotechnology Journal. https://doi.org/10.1016/j.csbj.2018.01.001 PMid:30275936 PMCid:PMC6158771
[41]
Vaisali, Parvathy, Vyshnavi, H., & Namboori, K. (2019). ' Tumor Hypoxia Diagnosis ' using deep CNN learning
strategy: A theranostic pharmacogenomic approach.
[42] Razmjooy, N., Estrela, V.V., Loschi, H.J. (2019). A survey of potatoes image segmentation based on
machine vision. In: Applications of Image Processing and Soft Computing Systems
in Agriculture. IGI Global, 1-38. 2019. doi:10.4018/978-1-5225-8027-0.ch001
[43] de Jesus MA, Estrela VV, Saotome O, Stutz D.
(2018). Super-resolution via particle swarm optimization variants. In: Hemanth
J., Balas V. (eds) Biologically Rationalized Computing Techniques For Image
Processing Applications. Lecture Notes in Computational Vision and
Biomechanics, vol 25. Springer, Cham doi: 10.1007/978-3-319-61316-1_14
[44] Hemanth, D.J., & Estrela, V.V. (2017). Deep
Learning for Image Processing Applications. Advances in Parallel Computing
Series, Vol. 31, IOS Press, ISBN 978-1-61499-821-1 (print), ISBN
978-1-61499-822-8 (online)
[45]
Xu, Y., Jia, Z., Wang, L., Ai, Y., Zhang, F., Lai, M., & Chang, E.I.
(2017). Large scale tissue histopathology image classification, segmentation,
and visualization via deep convolutional activation features. BMC Bioinformatics.
https://doi.org/10.1186/s12859-017-1685-x
[46]
Mistrangelo, M., & Lesca, A. (2013). PET-CT in anal cancer: Indications and
limits. In: Misciagna, S. (Ed.), Positron Emission Tomography - Recent
Developments in Instrumentation, Research and Clinical Oncological Practice.
IntechOpen. doi: 10.5772/57121. PMCid:PMC3593553
[47] Zacho,
H.D., et al. (2018). Prospective comparison of 68Ga-PSMA PET/CT, 18F-sodium
fluoride PET/CT and diffusion weighted-MRI at for the detection of bone
metastases in biochemically recurrent prostate cancer. European Journal of
Nuclear Medicine and Molecular Imaging, 45, 1884-1897. https://doi.org/10.1007/s00259-018-4058-4 PMid:29876619
[48] Voduc, D., Kenney, C., & Nielsen, T.O.
(2008). Tissue microarrays in clinical oncology. Seminars in Radiation
Oncology, 18 2, 89-97. https://doi.org/10.1016/j.semradonc.2007.10.006
PMid:18314063 PMCid:PMC2292098
[49] Mascini, N.E., Teunissen, J., Noorlag,
R., Willems, S.M., & Heeren, R.M. (2018). Tumor classification with
MALDI-MSI data of tissue microarrays: A case study. Methods, 151, 21-27 . https://doi.org/10.1016/j.ymeth.2018.04.004 PMid:29656077
[50] Alves, F.D., Estrela, V.V., & Matos, L.F.
(2011). Hyperspectral analysis of remotely sensed images. In: Sustainable Water
Management in the Tropics and Subtropics - And Case Studies in Brazil. Vol. 2,
University of Kassel. ISBN 978-85-63337-21-4
[51] Mezheyeuski, A., Bergsland, C.H., Backman, M.,
Djureinovic, D., Sjöblom, T., Bruun, J., & Micke, P. (2018). Multispectral
imaging for quantitative and compartment‐specific immune infiltrates reveals
distinct immune profiles that classify lung cancer patients. The J. Pathology,
244, 421-431. https://doi.org/10.1002/path.5026 PMid:29282718
[52] Ferro, A., Mestre, T., Carneiro, P., Sahumbaiev,
I., Seruca, R., & Sanches, J.M. (2017). Blue intensity matters for cell
cycle profiling in fluorescence DAPI-stained images. Laboratory Investigation,
97, 615-625. https://doi.org/10.1038/labinvest.2017.13 PMid:28263290
[53] Tasoglu,
S., Kavaz, D., Gurkan, U.A., Guven, S., Chen, P., Zheng, R., & Demirci, U.
(2012). Paramagnetic levitational assembly of hydrogels TIO. Adv. Mater. 25 (8)
1137. https://doi.org/10.1002/adma.201200285 PMid:23288557 PMCid:PMC3823061
[54] Asghar, W., Assal, R.E., Shafiee, H., Pitteri,
S.J., Paulmurugan, R., & Demirci, U. (2015). Engineering cancer
microenvironments for in vitro 3-D tumor models. Mat. Today. https://doi.org/10.1016/j.mattod.2015.05.002 PMid:28458612 PMCid:PMC5407188
[55] Rodell,
C.B., & Burdick, J.A. (2014). Materials science: Radicals promote magnetic
gel assembly. Nature 514 (7524) 574. https://doi.org/10.1038/514574a
PMid:25355357
[56] Zhou, Q., Vincent, M., Deng, Y., Yu, J.,
Xu, J., Xu, T., Tang, T., Bian, L., Wang, Y.J., Kostarelos, K., & Zhang, L.
(2017). Multifunctional biohybrid magnetite
microrobots for imaging-guided therapy. Science Robotics, 2.
https://doi.org/10.1126/scirobotics.aaq1155
[57] Mohammadzadeh N, Safdari R (2014). Robotic
surgery in cancer care: opportunities and challenges. Asian Pac J Cancer Prev 15:1081-1083.
https://doi.org/10.7314/APJCP.2014.15.3.1081 PMid:24606422
[58] Oblak, I., Češnjevar, M., Anžič, M., Hadžić,
J.B., Ermenc, A.S., Anderluh, F., Velenik, V., Jeromen, A., & Korošec, P.
(2016). The impact of anaemia on treatment outcome in patients with squamous
cell carcinoma of anal canal and anal margin. Radiology and oncology.
https://doi.org/10.1515/raon-2015-0015
[59] Norat, T., et al. (2005). Meat, fish, and
colorectal cancer risk: the European Prospective Investigation into cancer and
nutrition. J. Nat. Cancer Inst. 97 12, 906-16. https://doi.org/10.1093/jnci/dji164 PMid:15956652 PMCid:PMC1913932
[60]
Amirabdollahian, F., Livatino, S., Vahedi, B. et al. Prevalence of haptic
feedback in robot-mediated surgery: a systematic review of literature. J Robotic Surg (2018) 12: 11. https://doi.org/10.1007/s11701-017-0763-4 PMid:29196867
[61] Razmjooy, N., & Estrela, V.V. (2019). Applications of Image Processing and Soft Computing
Systems in Agriculture, IGI Global. doi: 10.4018/978-1-5225-8027-0
[62] Brodie, A.
(2018). The future of robotic surgery. Ann R Coll Surf Engl. 100(7), 4-13. https://doi.org/10.1308/rcsann.supp2.4 PMid:30179048 PMCid:PMC6216754
[63] Lhachemi, H., Malik, A., & Shorten, R.
(2019). augmented reality, cyber-physical systems, and feedback control for
additive manufacturing: A review. IEEE Access. 7, 750119 – 50135
https://doi.org/10.1109/ACCESS.2019.2907287
[64] Estrela, V.V., Monteiro, A.C.B., França, R.P.,
Iano, Y, Khelassi, A., & Razmjooy, N. (2019). Health 4.0: Applications,
management, technologies and review. Med Tech J, 2019;2(4):262-76. doi:
10.26415/2572-004X-vol2iss1p262-276. 262.
[65] Billah, M., Waheed, S., & Rahman, M.M.
(2017). An automatic gastrointestinal polyp detection system in video endoscopy
using fusion of color wavelet and convolutional neural network features. Int.
J. Biomedical Imaging. https://doi.org/10.1155/2017/9545920 PMid:28894460 PMCid:PMC5574296
[66] Estrela,
V.V., Coelho, A.M. (2013). State-of-the art motion estimation in the context of
3D TV. In: Multimedia Networking and Coding. IGI Global, 148-173.
doi:10.4018/978-1-4666-2660-7.ch006.
https://doi.org/10.4018/978-1-4666-2660-7.ch006
[67] Liang, H.,
Liang, W., Lei, Z., Liu, Z., Wang, W., He, J., Zeng, Y., Huang, W., Wang, M.,
Chen, Y., He, J., & Group, W.O. (2018). Three-dimensional versus
two-dimensional video-assisted endoscopic surgery: A meta-analysis of clinical
data. World Journal of Surgery, 42, 3658-3668. https://doi.org/10.1007/s00268-018-4681-z PMid:29946785
[68] Ito, Y.,
Ogawa, T., & Haseyama, M. (2017). Personalized video preference estimation
based on early fusion using multiple users' viewing behavior. 2017 IEEE
International Conference on Acoustics, Speech and Signal Processing (ICASSP),
3006-3010. https://doi.org/10.1109/ICASSP.2017.7952708
[69]
Cruz, B. F., de Assis, J. T., Estrela, V. V., & Khelassi, A. (2019). A compact SIFT-based strategy for visual information
retrieval in large image databases. Medical Technologies J., 3(2),
402-412, doi: 10.26415/2572-004X-vol3iss2p402-412.