Abnormal Skeletal Growth Patterns in Adolescent Idiopathic Scoliosis
Type of article: Original Article
h_kaced@univ-blida.dz
Abstract
Background: Adolescent Idiopathic Scoliosis
(AIS) occurs among children during their pubertal growth spurt. Although there
is no clear consensus on the difference in body height between AIS and healthy
controls, it is generally thought that the development and curve progression in
patients with AIS is closely associated with their growth rate.
Our aim is to compare the
anthropometric parameters of children with AIS and those of a control group within different age groups ranging
from 9 to 16 years old.
Methods: It is a prospective,
cross-sectional, case-control study which include 431children, 258 girls, 110
with AIS and 148 healthy controls, whereas in the group of males 173, 49 have
AIS and 124 don’t have deformity.
Anthropometric parameters, clinical
examination of the trunk and radiological assessment of the spine are records.
The statistical analysis is performed using SPSS package.
Children are examined from a
school-screening program in our physical medicine department in the university
hospital of Douera in Algiers. Measurements are assessed, including
anthropometric parameters (body height, body weight, secondary sexual
characters using Tanner stage, puberty age), trunk asymmetry and Cobb angle of
scoliosis.
Results: Girls with AIS are generally
taller, with a higher weight than the healthy controls with a significant
difference at the age of 12 years old. Otherwise, boys with AIS aged of 14
years are significantly taller than their controls.
Conclusion: The growth patterns in terms of
tallness with AIS are significantly different from healthy controls at the ages
of 12 for girls and 14 for boys.
Key words: scoliosis, screening, bone growth,
body height, body weight
Corresponding author: Dr Houria Kaced, Department of Physical Medicine and Rehabilitation,
University Hospital, DjillaliBounaama, Rue des frères halim, Douera, Algiers.
Blida1 University, Faculty of Medicine, BP 270 Route de Soumaa, Blida, Algeria.
Email: h_kaced@univ-blida.dz
Received: June 15, 2017, Accepted: October
30, 2017, English editing: November 27, 2017, Published: November 28, 2017.
Screened by iThenticate. ©2017KNOWLEDGE
KINGDOM PUBLISHING.
1. Introduction
AIS is known to be a three-dimensional spine deformity with unknown
pathogenesis, progression may occur until the end of bone maturity in 10% to
20% [10, 16, 22] of curves detected in school screening programs and not
treated. Factors that correlate with the risk of curve progression have been
identified in natural history studies of AIS, as sex, curves pattern, Cobb
angle, age at diagnosis, menarche and Risser sign [2, 4, 6, 18, 19, 21].
Many authors recognized that the development and progression of
idiopathic scoliosis are growth related and they reported that the curve
progression occurs during the adolescent growth spurt both in females and males
[6, 8, 9, 12, 17, 23]. More knowledge about this spine deformity revealed that
the pubertal development and curve progression in patients with AIS are closely
associated with their growth rate [1, 6, 7, 13, 20], as well as body growth
seems to be different between healthy children and those with idiopathic
scoliosis.
This correlation between growth and AIS was illustrated by the
Duval-Beaupère diagram (Fig.1) [8] which shows curves progression increasing
and coinciding with growth spurt during the peri-pubertal period, where height
velocity is the greatest at pubertal stages II and III of Tanner classification
[8, 17].
Classically, slowed aggravation continues until Risser 3-4 in girls
and later in boys at Risser5.
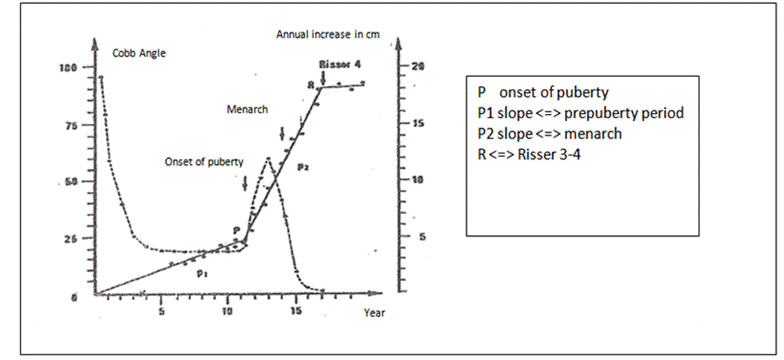
Fig 1:
Duval-Beaupère diagram [8] (translated from French to English).
This study aimed at comparing the
anthropometric parameters of children with adolescent idiopathic scoliosis
(AIS) and those of a control group with children age.
2. Material and Methods
We proceeded to a prospective study on the anthropometric
parameters of children with adolescent idiopathic scoliosis(AIS), using
cross-sectional and case-control data set in comparison with children age. We
performed this study within a school screening program managed during a 2-year
period between 2011 and 2012 at the department of physical medicine and
rehabilitation in Algiers, Algeria.
The inclusion criteria for patients were age and Cobb angle. School
children with ages ranging from 9 to 16 years were selected as it was
recommended in a study made in Algiers during 1995 and 1996 [11]. Patients
screened were considered having scoliosis, when the Cobb angle measured 10° or
more. For the control group we selected subjects without scoliosis that were of
similar age. Consent was obtained from all the parents before admission to the
study. Excluded were patients with evidence of abnormalities, thoracic
deformity, congenital spine abnormalities, skeletal dysplasia, neuromuscular
diseases and other types of scoliosis.
Different anthropometric parameters were assessed, using standard
procedures. Standing height was measured with the subjects standing upright
against a wall- mounted stadiometer, with their heads positioned in the
Frankfort horizontal plane and their heels against this tool.
Corrected height was calculated using Bjure equation: log y =
0.011x - 0.177[3, 14, 25], where y is the reduction in trunk height (cm) caused
by the spinal deformity, and x, the Cobb angle of the primary curve.
Body Weight (Kg) was measured in light clothes without shoes on a
standard weighing scale.
Body Mass Index was calculated considering the corrected height in
scoliotic school children.
Puberty was appreciated on Secondary Sexual Characters using
Tanner’s method [17] and menarche which age was 12.53 years for girls with AIS
and 12.97 for those without AIS. The difference was statistically not significant.
The period of changing (breaking) of voice was difficult to be known in boys.
The diagnosis of AIS was confirmed on a clinical examination using
Adam’s forward bending test [5, 11, 25], and a standard standing radiograph of
the Spine. The Adams test was done in ambient temperature, on undressed child.
The child bends at the hips to nearly 90° forward, with the arms relaxed, palms
together hand in front of the other, the knees straight, hind foot joint
together and forefoot making 30º. The physician inspects the trunk from a
posterior to anterior view, and notes any asymmetrical prominence on one side
of the thoracic or lumbar area, using a scoliometer.
Before this test was performed, we eliminated any pelvic tilt due
to leg length inequality. All children with trunk asymmetry received an X-ray
of the spine to confirm the diagnosis of scoliosis which is defined by Cobb
angle equal to 10° or more.
3. Results
We used the Statistical Package for the Social Sciences software
(SPSS Version 20.0) to calculate the Student’s t-test to compare two means. The
cut off mark of our level of significance is set to alpha equal to 5%.
Forty hundred and thirty-one (431) school children aged 9 to 16
years old were examined with a predominance of girls (59.86 %). 36.9 % of the
total presented the spinal deformity.
The distribution of AIS patients and their controls according to
their chronological ages is shown in the following graph (Fig. 2) where we see
that the girl’s sample is randomly distributed but not homogeneously.
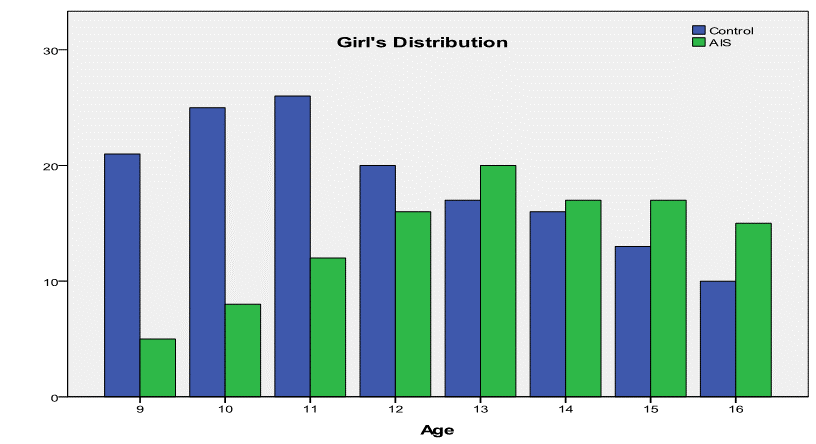
Fig.2: Comparison of girl’s
distribution between AIS and normal control sample.
That was different in boy’s population where the distribution was uniformly
homogeneous (Fig.3)
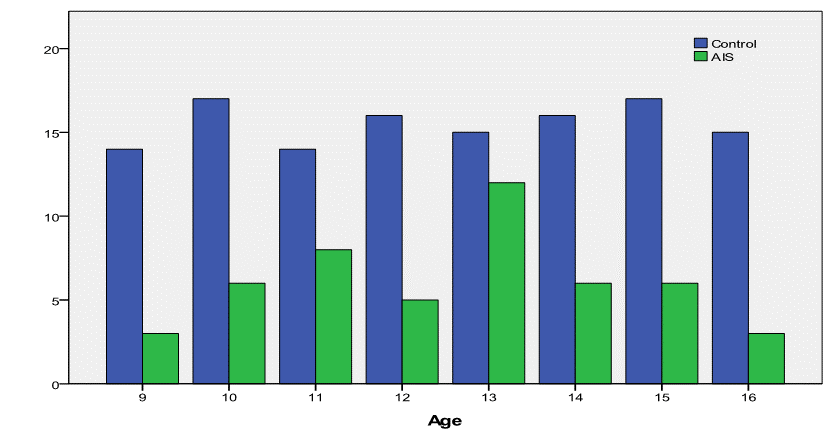
Fig.3: Comparison of boy’s
distribution between AIS and normal control sample.
Anthropometric Measurements, Girls
The
Body heights,
corrected heights, weights of girls with AIS and their healthy controls are
illustrated in the following graphs, respectively (Fig.4, 5, 6 and 7).
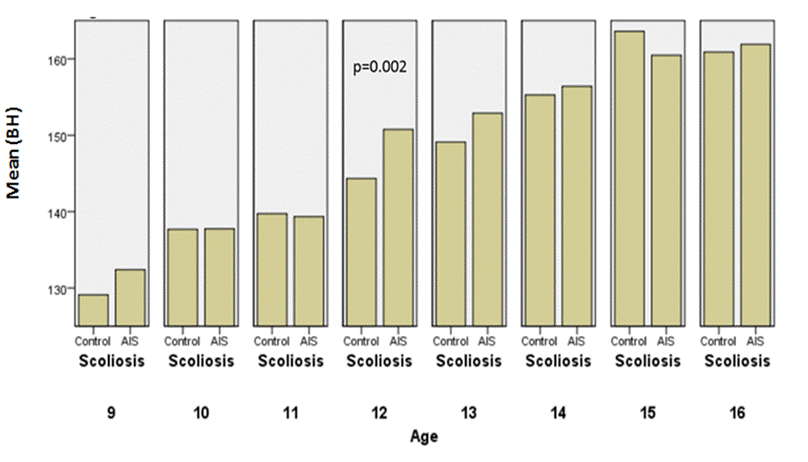
Fig.4: Comparison of uncorrected height between the controls and AIS by
chronological age in girls
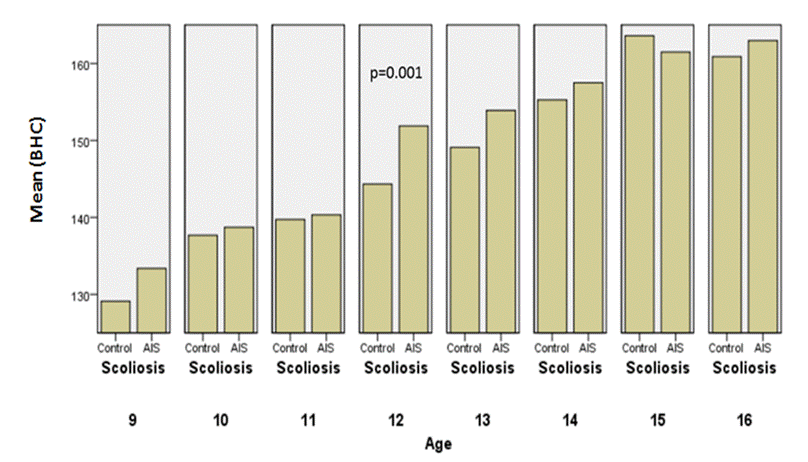
Fig.5: Comparison
of corrected height between the controls and AIS by chronological age in girls.
Girls with AIS were generally taller
than the healthy controls, considering uncorrected height
(p=0,002) and corrected body height
(p=0,001); height velocity was the greatest at the age 12 which corresponded to
the stages II and III of breast and pubic hair development (Tanner’s method).
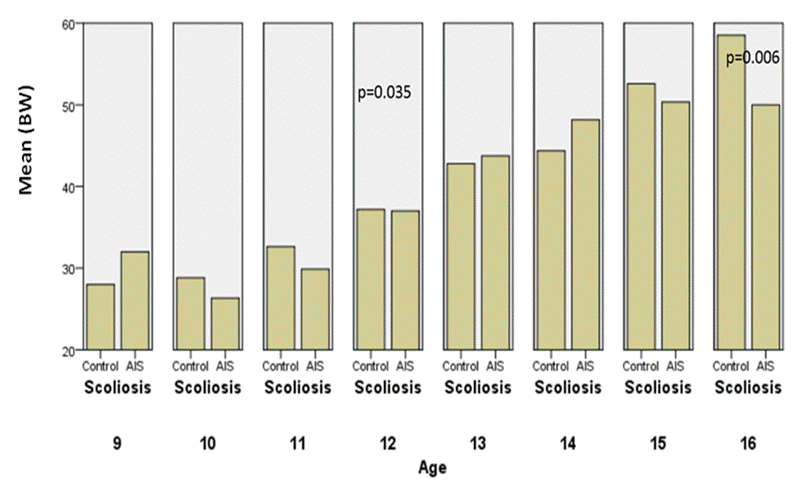
Fig.6. Comparisons of body weight between controls
and AIS by chronological age in girls.
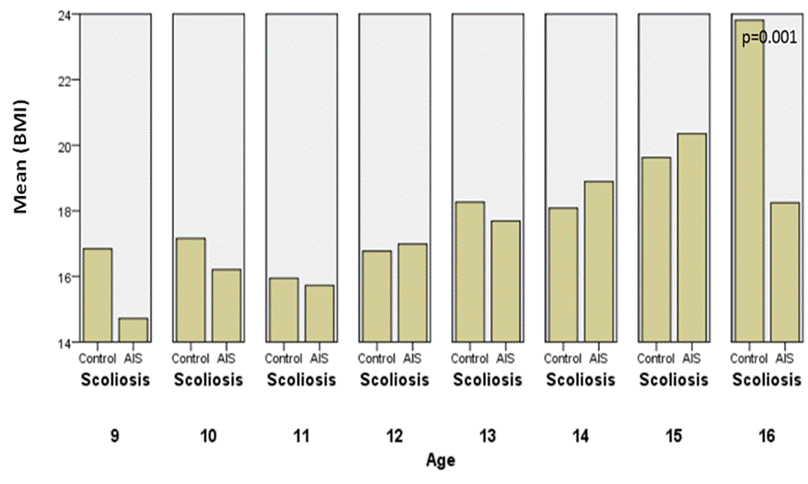
Fig.7.
Comparison of BMI between the controls and AIS by chronological age in girls.
Body weight is higher in AIS than the controls
at the onset of puberty with a significant difference, but at an age of 16
years they become underweight. As we see BMI is significantly different with
p=0.001.
All data obtained in girls are summarized in table 1
Table 1: Comparison
of anthropometric measurements between
female AIS and their controls by chronological age
|
Age (yrs) |
BH |
CBH |
BW |
BMI |
||||||||||
|
AIS |
Control |
p |
AIS |
Control |
p |
AIS |
Control |
p |
AIS |
Control |
p |
|
||
|
9 |
132±9 |
129±3 |
0 .458 |
133±3 |
129±9 |
0 .338 |
26±3 |
28±8 |
0 .562 |
15±1 |
17±3 |
0 .161 |
|
|
|
10 |
138±6 |
138±11 |
0 .982 |
139±11 |
138±6 |
0 .736 |
31±7 |
33±7 |
0 .663 |
16±2 |
17±3 |
0 .377 |
|
|
|
11 |
139±10 |
140±10 |
0 .907 |
140±10 |
140±10 |
0 .859 |
31±6 |
31±6 |
0 .961 |
16±2 |
16±2 |
0 .768 |
|
|
|
12 |
151±5 |
144±6 |
0 .002 |
152±7 |
144±5 |
0 .001 |
39±8 |
35±4 |
0 .035 |
17±2 |
17±2 |
0 .744 |
|
|
|
13 |
153±10 |
149±7 |
0 .205 |
154±7 |
149±10 |
0 .111 |
42±8 |
41±9 |
0 .661 |
18±2 |
18±3 |
0 .547 |
|
|
|
14 |
156±7 |
155±7 |
0 .629 |
158±7 |
155±7 |
0 .343 |
47±9 |
44±7 |
0 .263 |
19±3 |
18±2 |
0 .385 |
|
|
|
15 |
160±6 |
164±7 |
0 .205 |
161±7 |
164±6 |
0 .388 |
53±9 |
53±8 |
0 .915 |
20±4 |
20±3 |
0 .557 |
|
|
|
16 |
162±5 |
161±5 |
0 .635 |
163±5 |
161±5 |
0 .331 |
49±7 |
62±15 |
0 .006 |
18±2 |
24±5 |
0 .001 |
|
|
Anthropometric
measurements, boys
The following graphs (8, 9, 10
and11) illustrate the anthropometric measurements in boys.
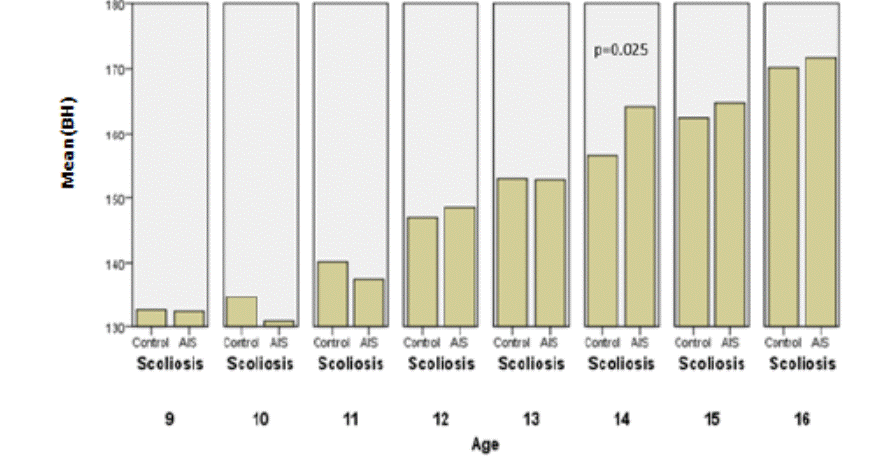
Fig.8: Comparison of height between the
controls and AIS by chronological age in boys.
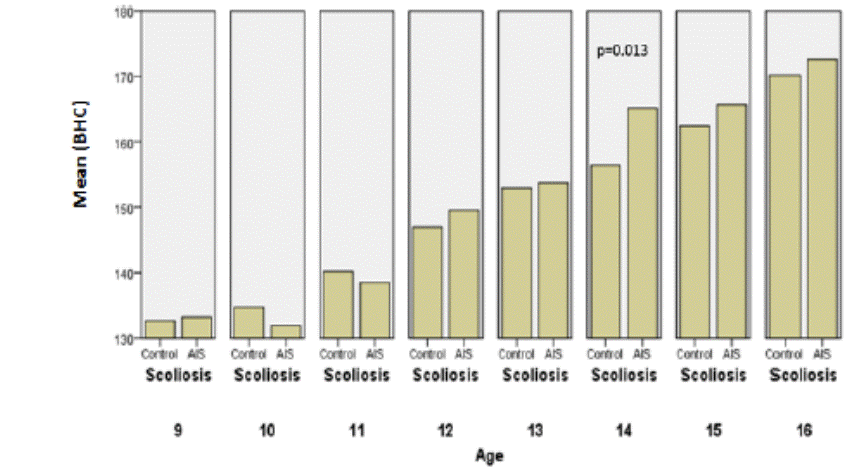
Fig.9: Comparison of corrected height between
the controls and AIS by chronological age in boys
In
terms of weight, we noticed that the BMI is higher at the age of 9 in AIS group
with p=0.042.
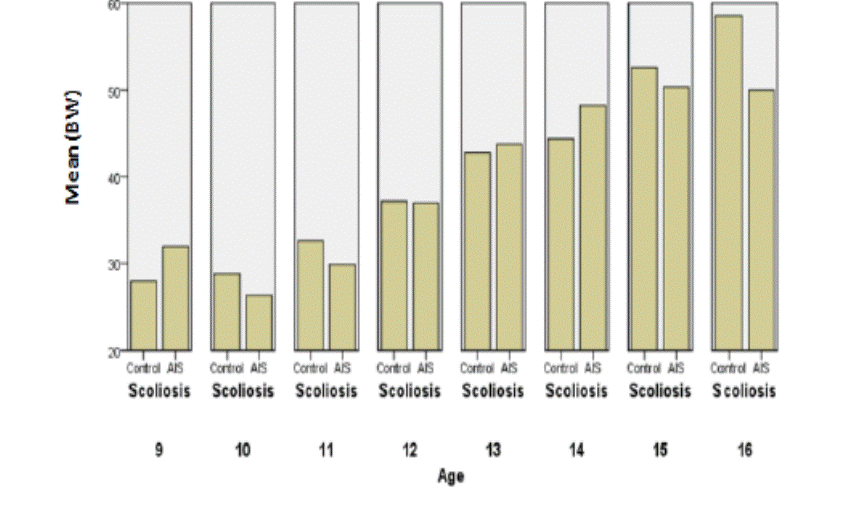
Fig.10: Comparisons of body weight between
the controls and AIS by chronological age in boys.
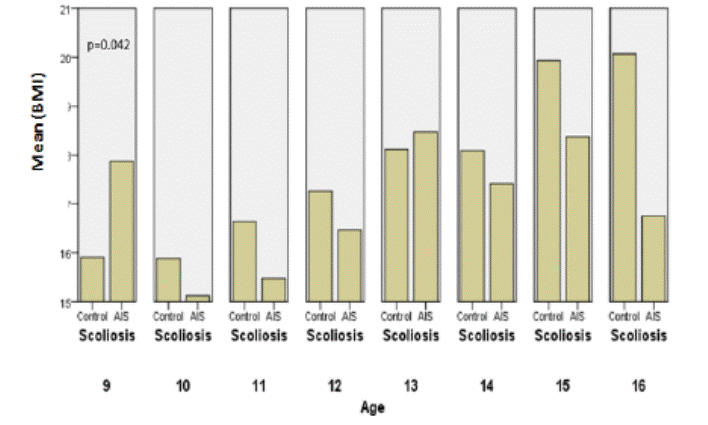
Fig. 11: Comparisons of BMI between the
controls and AIS by chronological age in boys.
All data
obtained in boys are summarized in table 2
Table2: Comparison of anthropometric measurements between
male AIS and their controls by chronological age.
|
Age (yrs) |
BH |
CBH |
BW |
BMI |
||||||||
|
AIS |
Control |
P |
AIS |
Control |
p |
AIS |
Control |
p |
AIS |
Control |
P |
|
|
9 |
132± 7 |
133±4 |
0 .935 |
133±7 |
133±4 |
0 .828 |
32±7 |
28±3 |
0 .107 |
18±2 |
16±1 |
0 .042 |
|
10 |
131±6 |
135±4 |
0 .104 |
132±6 |
135±4 |
0 .222 |
26±3 |
29±3 |
0 .079 |
15±1 |
16±1 |
0 .182 |
|
11 |
138±11 |
140±6 |
0 .482 |
138±11 |
140±6 |
0 .655 |
30±5 |
33±4 |
0 .205 |
15±1 |
17±2 |
0 .178 |
|
12 |
149±6 |
147±4 |
0 .537 |
150±6 |
147±4 |
0 .301 |
37±6 |
37±7 |
0 .957 |
16±2 |
17±3 |
0 .624 |
|
13 |
153±10 |
153±12 |
0 .973 |
154±10 |
153±12 |
0 .852 |
44±9 |
43±10 |
0 .808 |
18±4 |
18±3 |
0 .782 |
|
14 |
164±9 |
156±6 |
0 .025 |
165±9 |
156±6 |
0 .013 |
48±12 |
44±7 |
0 .349 |
17±2 |
18±2 |
0 .553 |
|
15 |
165±10 |
162±8 |
0 .577 |
166±10 |
162±8 |
0 .436 |
50±6 |
53±10 |
0 .607 |
18±2 |
20±4 |
0 .317 |
|
16 |
172±8 |
170±7 |
0 .736 |
173±8 |
170±7 |
0 .589 |
50±6 |
59±13 |
0 .288 |
17±1 |
20±3 |
0 .131 |
4. Discussion
Abnormal growth was observed in the
natural history of AIS during puberty as it has been reported in many important
studies [4, 6, 20, 23], which described more disorders in girls. In the present
study, the results demonstrate that the girls with AIS are generally taller
than the healthy controls, considering uncorrected and corrected height, the
difference is significant at the age of 12.
In the literature, Cheng and al [5] didn’t find any statistical
difference neither in uncorrected height nor in uncorrected sitting height
between AIS girls and normal controls at each age group except for the age of
15, however, after corrected trunk loss, girls with the spine deformity were
significantly taller than the controls between ages 13 and 15.Yim and al [25]
compared anthropometric parameters with severity of the curves and concluded
that, the uncorrected height was the same for each group of age and the
corrected height in AIS group with a Cobb angle greater than 40° was shorter
than the matched control at the age of 12, it subsequently caught up and became
significantly taller than the control group at the age of 14 to 16 years old
After analysis of data of weight, we see that girls with AIS are underweight
at an age of 16, and BMI was significantly lower with p=0.001. Certain authors [5,
25] reported that weight and BMI were lower in AIS than in controls, for Yim
and all other authors, it was significantly lower in the AIS20 and AIS40 groups
across all ages except for the age of 15 years.
Concerning boys, corrected and uncorrected heights are
significantly higher than matched controls at age of 14, while Wang who studied
arm spans and corrected standing heights showed that these measurements were
similar, in most of the ages [18].
Analysis of weights and BMI didn’t give us objective difference
between boys with AIS and the matched controls, even males seem to be
underweight at the end of maturity in the small sample of ours. When we compare
these results to the literature, we find that in a series larger than in our
study, Wang [18] demonstrated that male AIS presented lower body weights and
BMIs than their controls, between the ages of 15 and 17, with a significant
difference.
The present investigation, the first one in our country, aimed to
compare the anthropometric measurements between children with AIS and a healthy
control group of similar age during the peri-pubertal period in a small-scale
cross-sectional study of a school population sample.
Obviously, girls and boys with AIS exhibit abnormal longitudinal
growth. More than this we noticed in our empirical practice, that boys and
girls lost weight at the end of growth, but we can’t prove that. Indeed, we did not research about the
possible causes as genetic status, eating behavior, practicing sport, factors
that could influence growth.
We believe that, in addition to the anthropometric parameters which
are important maturity indicators that reflect growth and can predict the
progression of scoliosis curvatures, we must consider other signs such as
sexual characters, skeletal maturity (Risser sign, bone age) and morphology of
proper vertebral deformity especially in the sagittal plane that can contribute
to understand the worsening scoliosis.
More
investigation and more
research
in the field of spinal deformities will probably reveal that their progression
in children and adolescents depends on a set of known and less known factors,
and may be will highlight the relation between at last three elements as growth, genetics and nutritional status.
5.
Conflict of interest statement
We
certify that there is no conflict of interest with any financial organization
in the subject matter or materials discussed in this manuscript.
6.
Authors’ biography
No biography
7. References
1. Busscher I; Wapstra
FH; Veldhuizen AG. Predicting growth and curve progression in the individual
patient with adolescent idiopathic scoliosis: design of a prospective
longitudinal cohort study.BMC Musculoskeletal Disorders [BMC
MusculoskeletDisord] 2010 May 17; Vol. 11, pp. 93. https://doi.org/10.1186/1471-2474-11-93 PMid:20478013
PMCid:PMC2881883
2. Biondi J, Weiner DS,
Bethem D, Reed JF 3rd (1985) Correlation of Risser sign and bone age
determination in adolescent idiopathic scoliosis. J PediatrOrthop 5:697–701 https://doi.org/10.1097/01241398-198511000-00013 PMid:4066945
3. Bjure J, Nachemson A
(1973) Non-treated scoliosis. ClinOrthop Relat Res 93:44–52.
https://doi.org/10.1097/00003086-197306000-00007 PMid:4579097
4. Bunnel W.P. The
natural history of idiopathic scoliosis before skeletal maturity. Spine 1986;
11 (8): 773-6 https://doi.org/10.1097/00007632-198610000-00003
5. Cheng JC, Leung SS,
Lau J (1996) Anthropometric measurements and body proportions among Chinese
children. ClinOrthop. Relat Res 323:22–30 https://doi.org/10.1097/00003086-199602000-00004
PMid:8625584
6. Duval-Beaupère G.
Pathogenic relationship between scoliosis and growth. Scoliosis and growth.
Sorab, Livingstone, Edinbourg and London, 1971, 58.
7. Duval-Beaupère G.,
Barthel. F. La croissance des scoliotiques. Rev.Chr.Orthop. 1983; 69: 201-206
8. Duval-BeaupèreG . Les
lois d'évolutivité des scolioses. Application pratique. Réunion conjointe GES
et SRSQ. Montréal, 1979.
9. Goldberg CJ, Fogarty
EE, Moore DP, Dowling FE (1997) Scoliosis and developmental theory: adolescent
idiopathic scoliosis. Spine (Phila Pa 1976) 22:2228–2237 discussion 2237–2228
https://doi.org/10.1097/00007632-199710010-00006
10. Guillaumat.M. Lebard
J.P., Khoury N., Tassin J.L. Scoliose idiopathique en période de croissance. Encycl.Med.Chir
(Paris-France). AppareilLocomoteur, 15874 A10, 1991.
11. Kaced, H.
Belabbassi, H. School Screening for Scoliosis in Algiers. Results of a survey
conducted in 1995-1996. SOSORT 20-23 May, 2009; Lyon, France
12. Lonstein J.E.
Natural history and School Screening for Scoliosis. OrthopClin North Am 1988;
19: 227-37. PMid:3282198
13. Nachemson A.
Etiology and Natural History of Scoliosis. 1st European Congress on Scoliosis
and Kyphosis. Dubrovnik 1983
14. Sanders JO, Browne
RH, Cooney TE, Finegold DN, McConnellSJ, Margraf SA (2006) Correlates of the
peak height velocity in girls with idiopathic scoliosis. Spine (Phila Pa 1976)
31: 2289–2295
https://doi.org/10.1097/01.brs.0000236844.41595.26 PMid:16985455
15. Siu King Cheung C,
Tak Keung Lee W, Kit Tse Y, Ping Tang S, Man Lee K, Guo X, Qin L, Chun Yiu
Cheng J (2003) Abnormalperi-pubertal anthropometric measurements and growth
pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine
(Phila Pa 1976) 28:2152–2157
https://doi.org/10.1097/01.BRS.0000084265.15201.D5 PMid:14501928
16. Subileau. La
Scoliose, Dépistage Précoce. Journée du 2-10-1982. La Baule, France.
17. Tanner J.M. Growth
at Adolescence. Blackwell Scientific Publications, ed., Oxford, 2° ed., 1962
18. Wang Wei-Jun • Sun
Xu • Wang Zhi-Wei, QiuXu-sheng • Liu Zhen • Qiu Yong. Abnormal anthropometric
measurements and growth pattern in male adolescent idiopathic scoliosis Eur. Spine
J. (2012) 21:77–83 https://doi.org/10.1007/s00586-011-1960-x PMid:21826498 PMCid:PMC3252435
19. Weinstein SL,
Ponseti IV (1983) Curve progression in idiopathic scoliosis. J Bone JtSurg Am
65:447–455 https://doi.org/10.2106/00004623-198365040-00004
20. Weinstein S.L.
Idiopathic Scoliosis. Natural history. Spine 1986; 11 (8): 780 - 783.
https://doi.org/10.1097/00007632-198610000-00006
21. Willner S (1974)
Growth in height of children with scoliosis. ActaOrthopScand 45:854–866
https://doi.org/10.3109/17453677408989696
22. Winter R.B.
Adolescent IdiopathicScoliosis. N. Engl. J. Med.314, 1379 - 1380.
https://doi.org/10.1056/NEJM198605223142108 PMid:3702944
23. Winter R.B, Banta
J.V, Engler G. Screening for Scoliosis (Letter) JAMA 1995; 273:185 – 186
https://doi.org/10.1001/jama.273.3.185 PMid:7807649
24. Yasuo Y, Takuo Y,
and Yoshiyuki A. Prediction of Curve Progression in Idiopathic Scoliosis based
on Initial Roentgenograms. A Proposal of an Equation. Spine 1988; 13 (11): 1258
- 1261.
https://doi.org/10.1097/00007632-198811000-00009
25. Yim Annie P. Y,
Yeung Hiu-Yan, Hung Vivian W. Y, Kwong-Man Lee, Tsz-Ping Lam, Yong Qiu, Jack C.
Y. Cheng. Abnormal Skeletal Growth Patterns in Adolescent Idiopathic Scoliosis.
A Longitudinal Study until Skeletal Maturity. Spine. 2012;37 (18):E1148-E1154
https://doi.org/10.1097/BRS.0b013e31825c036d PMid:22565390