Recent Advances of Mechanical Engineering Applications
in Medicine & Biology
Type of article: review
Abdelkadir Belhadj1, Hadjer Boujemaa2
1Computational Mechanics Laboratory, Department
of Mechanics, Faculty of Technology, University of Tlemcen, Tlemcen, Algeria
2Laboratory of Natural Bioresources, Department of Biology, Faculty of Science,
Hassiba Ben Bouali University Chlef, Box 151, 02000 Chlef, Algeria.
Abstract
Background: Mechanics is an area of science dealing with the
behavior of physical bodies (solids and fluids) undergoing action of forces, it
comprised of statics, kinetics and kinematics. The advances and research in
Applied Mechanics has wide application in almost fields of study including
medicine and biology. In this paper, the relationship between mechanical
engineering and medicine and biological sciences is investigated based on its
application in these two sacred fields. Some emergent mechanical techniques
applied in medical sciences and practices are presented.
Methods: Emerging applications of mechanical engineering in
medical and biological sciences are presented and investigated including:
biomechanics, nanomechanics and computational fluid dynamics (CFD).
Results: This review article presents some recent advances of
mechanical engineering applications in medicine and biology. Specifically, this
work focuses on three major subjects of interests:
- Biomechanics that is
increasingly being recognized as an important application of mechanical
fundamentals in biomedical and biological sciences and practices,
biomechanics can play a crucial role in both injury prevention as well as
performance enhancement of living systems.
- Novel techniques of
nanomechanics including: Carbon nanotubes
applications in therapy, DNA recognition, immunology and antiviral
resistance. Nanorobotics that combines between nanotechnology, mechanics
and new biomaterials to design and develop nanorobots based bacteria and
biochips; these nanoscale robots can be involved in biomedical
applications, particularly for the treatment of cancer, cerebral aneurysm
treatment, kidney stones removal surgery, treatment of pathology,
elimination of defected parts in the DNA structure, and some other
treatments to save human lives.
- Computational fluid
dynamics (CFD) tools that contribute on the understanding of blood flows,
human organs dynamics and surgical options simulation.
Conclusion: Recent advances of mechanical applications in
medicine and biology are carried out in this review, such as biomechanics,
nanomechanics and computational fluid dynamics (CFD). As perspectives,
mechanical scholars and engineers can involve these cited applications in their
researches to solve many problems and issues that doctors and biologists
cannot.
Keywords: Biomechanics, Nanorobotics, Medicine, Biology,
Biomedical engineering.
|
Corresponding
author: Dr
Abdelkadir Belhadj, Computational Mechanics Laboratory, Department of
Mechanics, Faculty of Technology,
University of Tlemcen, Tlemcen, Algeria Email: belhabdelkadir@gmail.com Received: July
28, 2017, Accepted: September 27, 2017, English editing: September 27, 2017,
Published: September 28, 2017. Screened by
iThenticate. ©2017 KNOWLEDGE KINGDOM PUBLISHING. |
1. Introduction
As applied
physics, the modern mechanical engineering which permeates almost all the core
Engineering or rather scientific disciplines, has proven its reliability to be
involved in resolution of complex problems in several disciplines even medicine
and biology.
Why does
mechanics serve for medical technologies and biological sciences?
Mechanical
engineering is a broad engineering subject with a range of activities and
functions that derives its breadth from the need to design and manufacture
medical technologies from small individual parts and devices to large systems that
can be involved in almost every aspect of technology. It covers topics related
to energy, fluid mechanics and dynamics, robotics, solid mechanics, heat
transfer, design and manufacturing, maintenance and control. This diverse
background helps mechanical engineers and scholars to define, orient the future
of technology, and play a critical role in solving global issues and challenges
of many areas of interest outside mechanical technologies. Medicine and
biological sciences have been adopted by mechanical principles and theories
such as fundamental role in orthopedics, immunology, or the absolute reliance
of mass transport and diffusivity equations on pharmacokinetics and
pharmacodynamics for understanding cardiovascular physiology and pathology.
Currently, the meeting between mechanical engineering and medicine oversteps
than what were unimaginable until recent times, because of the integration of
novel disciplines and novel techniques. We can cite many topics and emergent
issues including engineering mechanisms, processes, bio-sensors and bio-devices
in medicine, biology and healthcare, where the mechanics is the main player and
the key for problem-solving. Following are some topics that connect mechanics
with medicine and biology:
• Biofluid Mechanics, Biorheology, Blood Flow
dynamics
• Hemodynamics using Computational Fluid
Dynamics (CFD).
• Biomaterials and Biosensing
• Cellular, Subcellular, Genetic, Epigenetic,
or Molecular Biomechanics
• Medical Nanoelectro-mechanical Systems (NEMS)
• Medical Robotics.
• Reproductive and Urogynecological Mechanics.
• Muscle/Neuromuscular/Musculoskeletal
Mechanics and Engineering.
• NEMS/MEMS, Microfluidics.
• Mechanobiology and healthcare
• Computational Biomechanics/Physiological
Modelling
• Clinical Biomechanics.
• Cellular and Tissue Mechanics/Engineering.
• Cardiovascular/Cardiac Mechanics.
• Cardiovascular Systems Engineering.
• Bio-Nanotechnology and Clinical Application.
• Biomedical Signal Processing Techniques
• Artificial Organs, Biomechanics of Organs.
• Medical Instrumentation and BioSensors.
• Respiratory System Engineering.
• Bioheat Transfer and Mass Transport, Nano
Heat Transfer.
• Human Movement and Animal Locomotion.
• Implant Design and Mechanics.
• Sports Medical Mechanics, Joint Mechanics.
• Therapeutic Physics and Rehabilitation
Engineering.
2. Biomechanics applications
Biomechanics is
the application of mechanical principles in the study of living organisms
including their kinematics (description of motion) and kinetics (actions of
forces associated with motion), it views the human body as a collection of
levers, made of bones which are moved by its muscles. In sport and exercise,
where mechanics can be involved to analyze the performance of athletes based on
their interaction with the equipment. [1]. Figure.1 presents a case study of a
knee joint simulated via Ansys.
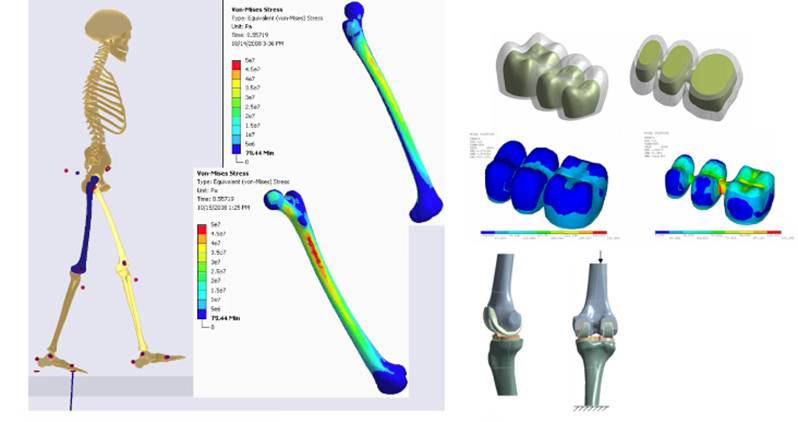
Figure.1 Musculoskeletal model coupled with
ANSYS allows simulation of femur stresses during gait, residual viscoelastic
stresses in a partial denture, knee joint geometry for wear simulation. [2]
According to the
scale in which the study or the application is done, we can distinguish between
biomechanics and mechanobiology. Biomechanics is more related to the scale of
body segments, interaction with the surrounding environment, etc. On the other hand,
Mechanobiology is concerned more with the level of cells, it focuses on the
physical forces behavior and transfer in cell or tissue mechanics.
3. Nanomechanics
applications:
Nanotechnology is the understanding the
behavior of matter at infinitesimal dimensions called nanometers (a nanometer
is one-billionth of a meter; a human hair is about 75000 nanometers in diameter),
where incredible properties enable emergent applications. Considering combination
between nanoscale science, engineering and technology, nanotechnology covers
sensing, imaging, measuring, manufacturing, control and manipulating nanoscale
matter. In mechanics, the integration of nanotechnology is focused on three
main topics including: nanostructures (carbon nanotubes), nanofluids and
microfluidics, and nanorobotics. In following, we present the application of
these nanomechanics topics in medicine and biology.
3.1. Carbon nanotubes:
Carbon nanotubes (CNTs) [3] are nanoscale structures
made of pure carbon that are long and thin and shaped like tubes, these
molecules are same sized and structured in chemical boding and aligned by Van
der Walls forces into ropes, the length
of CNTs can reach some millimeters while its diameter in on the order of some
nanometers. In reference to the number of structured walls, we can distinguish
single walled nanotubes (SWCNTs) shown in Figure.2, and multi-walled nanotubes
(MWNTs) depending upon the walls number.

Figure.2 CNT molecular diagram
In addition to spherical bucky-balls,
nanotubes are also members of the fullerene structural family, named nanotubes
from their long length and hollow structure formed by the turning of single
atom thick sheets of a matter called graphene, which is extracted from graphite.
The importance of CNT’s are their exceptional electrical, mechanical [4],
optical and chemical properties. The use of CNT’s in medicine and biology is
presented as following:
3.1.1. The application of
carbon nanotubes in cancer therapy
Cancer belongs to the most complicated
diseases in the world. According to the WHO, the cancer is considered one of
the main causes of morbidity and mortality worldwide, with approximately 14
million cases in 2012 [5]. Anticancer drugs like Chemotherapy or Radiotherapy
often have physiological, biochemical and cellular toxic side effects. Several
methods in many fields have been objected to reduce this problem, among them,
Carbon Nanotubes (CNTs). They have unique mechanical properties that open a way
for many therapeuticsto strongly minimize their side effects. Many Centers for
Cancer Research focus to discover new drugs originating from Carbon Nanotubes.
3.1.2. The anticancer
agent taxoid with a cleavable linker
Taxoid is a chemotherapeutic agent to block
proliferating cancer cells. CNTs have been explored as a tool in nanocarriers
for the exploration of novel drugs. There are large varieties of nanoscale drug
delivery vectors like single-walled carbon nanotubes (SWCNTs). As CNTs are
needle-like shape, they have been involved in injection and integration into
target cells [6], CNTs are combined to the anticancer agent taxoid as a
cleavable linker [7]. In order to ensure the target cell, the drug is
transported via endocytosis and released in the cell. Microtubules interact
with the drug as evaluated by flow cytometry thus formatting a stable
microtubule-taxoid complex. [7].
3.1.3. Target drug
delivery for cancer therapy
One of the novel applications of CNTs is
drug delivery called also smart drug delivery, a method of high recognition of
cancer cells or cancer tissues [7] in order to deliver medication with high
precision. It is efficient for the lymphatic system; metastases of certain
cancers can be effectively inhibited [8] for subcutaneous injection. Adsorption
on the PAA-CNT surface [9] is possible through coprecipitation of Fe3O4-based magnetic nanoparticles,
polyacrylic acid (PAA) can be added to CNTs to become highly hydrolic.
3.1.4. The “longboat”
anticancer system
In this application, CNTS are used for
cancer treatment based on a functionalized SWNT attached to a complex of
cisplatin and folic acid derivative via covalent or noncovalent bonding to comprise the “longboat” which has been
reported to be taken up by cancer cells via endocytosis; then, the release of
the drug and its interaction with the DNA. Targeted single-wall carbon
nanotube-mediated Pt(IV) prodrug delivery is using folate as a homing device [10]
3.1.5. The application of
carbon nanotubes in cancer immunotherapy as a vaccine
Nanotechnology has advanced in theoretical
and practical research in all fields of biomedicine.Recently, the application
of Nanotechnology in Immunotherapy has opened another choice for the treatment
of cancer, which was evaluated as an
anticancer drug by using CNTs. Carbon nanotube antibodies are used to recognize
and target tumor cells. Many works have been done to show the possibility the
anticancer immune reaction increase of tumor cell by using CNTs as delivery media.
Ruggiero et al [11] have reduced the tumor volume by creating complexes of
tumor neovascular-targeting antibody E4G10 to SWCNTs using radiometal-ion
chelates and improved median survival time relative to control [12]. Fan et al
[13] haveconfirmed that intracranial CNT–CpG therapy blocked subcutaneous
melanomas. Recently, Fadel et al [14] attached antigens to bundled CNTs
(CNT–polymer composite). This CNT complex was conjugated with polymer NPs
containing magnetite with T-cell growth
factor IL-2. The results proved that T-cells denied the tumor growth.
3.1.6. Stimulation of
immune system
Using oxidized Multiwall Carbon Nanotubes
(MWCNT), the immune system activity increased in a hepatocarcinoma
tumor-bearing mice model. After injection of CNTs the activities of immune
cells were stimulated by activation and stimulation of phagocytosis of
macrophagesand promotion of inflammatory cytokines secreted due to the
activation of the complement system [15].
3.1.7. Using complex
specific IgG responses for antigen stimulation
Villa et al. [16] have demonstrated that
SWCNTs can activate humoral immune responses. There are studies about the
possibility ofenhancement of immune responses by using SWCNTs as antigen
carriers. A complex of a number of peptides (0.4 mmol/g) and SWCNTs was created
and internalized into professional APCs . This created specific IgG responses
against the peptide,
3.1.8. T-cell
(Treg)-specific receptors
A group of researchers provides a
foundation for innovative immunotherapy against cancer; they investigated in
vivo the selective internalization of Polyethylene Glycol-modified SWCNTs
(PEG–SWCNTs) to be drived by ligands against T-cell (Treg)-specific receptors
in the tumor microenvironment. Whereas, PEG–SWCNTs with glucocorticoid-induced
TNFR-related receptor GITR ligands were internalized by Treg through
receptor-mediated endocytosis and conveyed into the cytoplasm/nucleus cytoplasm
and nucleus ex vivo and in vivo [17].
Recently, Fadel et al. [14] demonstrated
that T-cells suppressed tumor growth.
asntigens were combined to bundled CNTs (CNT–polymer composite) and this CNT
complex was attached to polymer NPs that contains magnetite and the T-cell
growth factor IL-2.
3.1.9. Vaccine
Creation of vaccine at the stimulation of
immunity against a tumor cell employs the association of MWCNTs to tumor lysate
protein. An
efficient tumor curing and a cellular antitumor immune reaction is improved
[15] in an H22 liver cancer-bearing mice. The antitumor immune reaction
response was specific. The antibody delivery system in immunotherapy was
ensured by CNTs that can be used to promote new antitumor immunotherapies.
3.1.10. The application
of carbon nanotubes in infection therapy
The gradual emergence of resistant
bacteria is occurring worldwide have enhanced medicine and saved many people-threatening
bacterial infections [18]. In the past, pharmaceutical antibiotics have been
used as a strategy to combat resistant bacteria. Currently, the use of
innovative antimicrobial agents [19] for infection therapy has been answered by
CNTs that serve as novel antibiotics for the treatment of these infections.
3.1.11. Application of
carbon nanotubes for attacking antibacterial resistance drugs
The carbon nanotubes have been evaluated
for infection therapy to attack multi drug resistant bacteria [20]. The effect
of benign ε-polylysine/silver nanoparticle, nanocomposite
(EPL-g-butyl@AgNPs) with polyvalent and synergistic antibacterial is reported
to understand the antibacterial mechanism of AgNPs-based nanocomposites, and
devrlop an efficient antibacterial agents
for clinical applications.
3.1.12. Application of
carbon nanotubes for attacking fungi
Functionalized CNTs have been studied like
the antifungal amphotericin. After crating, a complex with association of CNTs
and amphotericin B this was transported it into mammalian cells. This conjugate
has reduced the antifungal toxicity [21].
Combination of the antimicrobial agent Pazufloxacin mediated with
amino-MWCNT demonstrated a high adsorption and will be applied to experimental
assays for infection treatment [22].
3.1.13. Application of
carbon nanotubes for attacking antiviral resistance
Wang et al. [23] have evaluated the
adsorption behavior of the antiviral drugs oseltamivir (OE) and its metabolites
(i.e., oseltamivir carboxylate (OC) on CNTs, three types of CNTs are used
including SWCNTs, MWCNTs and carboxylated SWCNT (SWCNT-COOH). The comparison of
the adsorption on different CNTs shows that SWCNTs-COOH plays a key role during
the adsorption process. The adsorptive mechanism of hydrophobic interaction
electrostatic interaction, Van der Walls force and H-bonding were suggested as
the contributing factors for OE and OC adsorption on CNTs. Especially, to
affirm the contribution of electrostatic interaction; the changes of adsorption
partition performance (Kd) of OE and OC on CNTs were evaluated by varying pH
from 2 to 11 and the importance of isoelectric point (pHIEP) of CNTs on OE and
OC adsorption was addressed.
3.1.14. Carbon Nanotubes
for Gene Therapy by DNA Delivery
Gene therapy by DNA Delivery using CNTs has
spread out. Curently around 4000 genetic disorders are identified. Almost if
not most of them are hereditary and caused by mutations [24]. Gene therapy is
regarded as a new technique that deal with several incurable morbus, such as
cancer and other genetic disorders [25]. The use of carbon nanotubes
substitution of mutated genesis targeting at acquainting DNA molecule into the
cell nucleus. Whereby a broaden range of therapeutically active nucleic acids,
including plasmid DNA (pDNA), small -interfering RNA (si RNA) , antisene oligo
Deoscy Nucleotides (ODNs) , and aptamers, have experiense at the
posttranscriptional or translational levels Kun Him [12]. Ramos -Perez [26.]
designed the surface modification of carbon nanotubes (CNTs) to permit the
formation of a complex between these potential carriers with DNA. Procedures
were developed to prepare transfection vectors through the modification of
MWCNTs. These procedures composed of divisions determined the reduction of CNTs
length, to rise the dipersability of CNTs and finally for a surface
modification to attach through electrostatic interaction DNA to the CNTs.
Several f-CNTs have been studied to deliver p DNA using amine groups,
polyethylenemine hybrids, cationic, glycopolymers, and ethylenediamine. Singh et
al [27] underseek the optimization of f-CNTs as Gene delivery vehicles,
including ammonium- funti onalized MWCNTs ( MWCNTs-NH3+, SWCNTs - NH3+) and
lysine functionalized SWCNTs (SWCNTs–lysine–NH3+), with pDNA resulting in a complex formation between f-CNTs and DNA. Pantarotto et al [28] offered an
ammonium-functionalized SWCNTs with pDNA to reduce cytotoxicity. The gene
expression level by f-CNT-based DNA delivery was tenfold higher.
3.2.
Nanorobotics:
Nanorobotics has been widely known as an
emerging field developing small machines and devices in the scale of some
micrometers involved in nanobiotechnology, using specific materials to build
what called nanorobts. These nanorobots (nanobots) are applied in microbiology
as an effective strategy by enabling propulsive potential by attaching them to
magnetotactic bacteria (magnetococcus, magnetospirillum, magnetotacticum and
magnetospirillum magneticum [29]) .
Using the application of magnetic field [30] to guide these bacteria to follow
a desired direction (Target cells).
Another application for these micro/nanodevices as smart sensors [31] to
collect information. In surgery and medical treatments, microdevices has
brought many in clinical procedures for heart and intracranial surgery [32-34],
pervasive medicine [35, 36], and medical procedures [37, 38]. Figure.3 shows a
nanorobot with a human embryo.
The use of nanorobots in
medicine has been widely emerged and impacted; the injection of nanorobots in
human body has been carried on for several purpose such as, imaging, sensoring,
measuring, cleaning up, surgery and delivering differentiated stem cells… Nonetheless,
the future hides surprises to us, maybe in few years nanorobots will replace
our organs as they wear out.
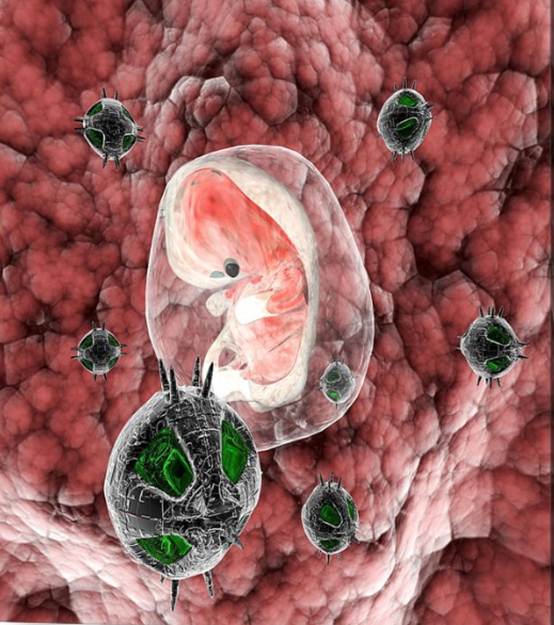
Figure.3 Nanorobots with human
embryo by Christian Darkin [39]
Drug delivery is also ensured by using
specific nanorobots called Pharmacytes, where the dosage of drug is loaded in
to its payload. Pharmacytes ensure the precision in transport of drug delivery to specific
cellular targets [40].
Currently, research is focused to design a nanorobot dubbed as
respirocyte. The function of the microrobot is linked with the bloodstream
physiology. First, collecting oxygen as it passes through the respiratory system
via blood circulation system. Second, collecting carbon dioxide from tissues
for release into the lungs. Then, metabolizing glucose to power its own
functions [41]; The application of nanorobotics in Hemostasis is an emerging
smart process involving several steps with a number of promoters and inhibitors
balancing thrombosis and fibrinolysis [42].
In neurosurgery, in order to reduce the
average of mortality, nanorobots are used in diverse ways for screening for a
new aneurysm or closer monitoring of an identified aneurysm. In this issue, Cacalcanti
et al. [43] have proposed a novel design for an intravascular nanorobot with
the specific property to detect aneurysm formation by detecting the increase of
nitric oxide synthase protein levels within the affected blood vessel.
3.3. Nanofluids:
Nanofluid
is a fluid mixing nanoscale solid particles,
called nanoparticles. The use of nanofluids and dispersant nanoparticles in
biology is widely investigated [44]. These fluids
are engineered colloidal suspensions of nanoparticles in a base fluid.
The aim purpose to use these nanosolids is to benefits from its thermal
properties for heat and mass transfer, or electrical and magnetic properties
for power transmission or sensing. Nanoparticles which are commonly used in
nanofluids are made from numerous materials such
as oxide ceramics (Al2O3,CuO) [45]. In
recent years, Most of the application of nanofluids in biomedicine were
fluorescent biological labels [46], drug and gene delivery [47],bio detection
of pathogens [48], detection of proteins [49], probing of DNA structure [50]
and tissue engineering [51]. Nanoparticles are on the way to become an extensive field of interest
worldwide. The nanoscale has several properties and varieties like size, shape,
and diverse components to permit explorations for biomedical applications.
Currently, intensive research is done to
be applied for the implantation of tissues or the search for cancer
therapeutics.
It is aimed at augmenting the chance of
compatibility and acceptance of implanted tissues, to reduce the chances of
rejection as well as to stimulate the production of osteoblasts by creating
nano-sized features on the surface of e hip or knee prostheses. A 3D analysis
based on optical "bar coding" of polymer particles in solution, is
limited only by the number of unique tags one can reliably produce and detect.
Single quantum dots of compound
semiconductors were successfully used as a replacement of organic dyes in
various bio-tagging applications. A precise control of quantum dot ratios has
been achieved. The selection of nanoparticles used in those experiments had 6
different colors as well as 10 intensities. It is enough to encode over 1
million combinations [52].
An Italian group presented a study with the
application of nanoparticles in cell therapy for myocardial infarction
treatment and heart regeneration. They focused on the traditional approach to
deliver cells at the damaged site [53]. Another group seeks to develop antibodies using conjugated
fluorescent dye-doped silica nanoparticles (FDS-NPs) for the rapid detection of
Salmonella spp. [54].
Costescu et al. [55] aim to
evaluate nanoparticles (Ag:Hap-NPs) for their antibacterial and antifungal
activities, using pure silver-doped
nanocrystalline hydroxyapatite nanoparticles.
4. Computational Fluid
Dynamics:
Computational Fluid Dynamics is an
engineering tool that connects mechanics to mathematics and software
programming to execute simulation performing how a fluid (liquid or gas) flows
based on Navier-Stokes equations which are the main mathematical formulation
modelling all phenomena of fluid mechanics. The solution of these equations is
elaborated by implementing structured and unstructured meshes using numerical
methods such as (finite volume method, and finite element method). CFD has been around since the early 20th century as a
tool analyzing air flows around cars, aircraft and performing the cooling
systems of data centers and electronic chips. CFD softwares like Ansys,
Solidworks, Openfoam, ADINA… are playing a key role in medecine andbiology,
where researchers create virtual reconstructions of different human organs
[56], surgical options [57] and blood flow [58] system, combining fluid
dynamics results with a simplified model of the human body such as the vascular
(figure.4) and pulmonary systems. Simulations can actually predict blood flow
distribution across the arteries (figure.5) and energy losses at the possible
surgical connections.
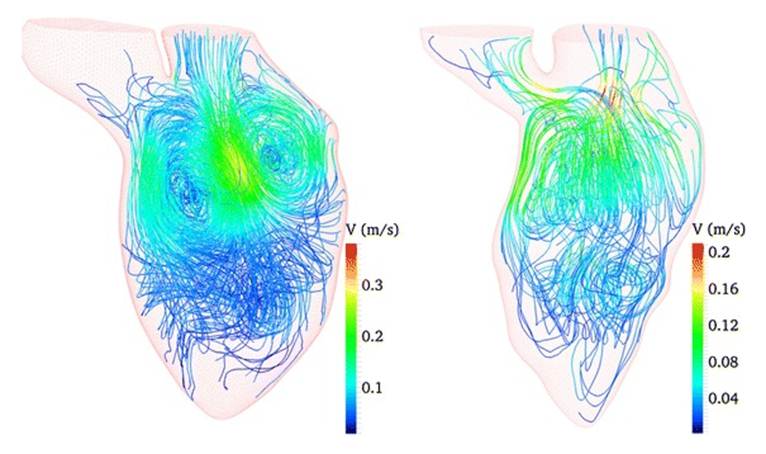
Figure.4 Exemple of CFD simulation of heart blood flow [59]

Figure.5 Exemple of CFD simulation of
arterial microanastomoses [60]: (i) anatomy of the sdistal femoral artery, (ii)
view prior to anastomosing, (iii) a completed anasotomosis,
5. Evaluation and Discussion
of presented applications:
The objectives of this study are to
critically evaluate these technologies, promote them as emerging areas of
research and development for mechanical engineers and scholars, and built a
real partnership between medicine, biology and mechanics.
In this review, some recent advances of
mechanical engineering applications have been presented summarized in three
main topics:
- Biomechanics which ranges from the inner working of cell to the
moving forces that acts on limbs, this field of study is the most
investigated by mechanical engineers and scholars among other mechanical
technologies. In recent years, many efforts have been devoted to enhance
biomechanical research and innovation to advance the field of tissue
engineering a well as sport biomechanics and bio mechanobiology.
- Nanomechanics that studies the nanoscale machinery and fluidics, is
one of the emerging topics in science and technology in the last decade.
Research and development in this field focuses on the introduction of
nanorobotics in surgery and medical treatment. Researches done by
physicians in this field need to be more audacious and creative.
Nanomaterials based CNT’s are expected to play an important role in
medicine and biology improving the way we live by biological diagnostics
using biosensors to recognize molecules and organs, the mechanical,
optical, thermal, electrical and chemical properties of CNT’s make them in
addition to silicon nanowires the building materials for DNA delivery and
proteins sensing. Therefore, research in this field is very promising for
mechanical scholars.
- CFD tools to execute some simulations for blood flows,
cardiovascular pumping and inertia, surgical procedure and external effect
on human body. Because of the experimental data are not available, CFD
simulation are more theoretic, the collaboration between medical
scientists and physicians is required to conduct real-time simulations and
achieve coherent results.
Mechanical engineers and scholars can
refer to this review to have an overview about the recent advances of
mechanical application in medicine and biology, and try to orient their studies
to this issue.
6. Concluding Remarks:
This paper gives an overview to describe
and understand some applications of mechanical engineering and sciences in
medicine and biological sciences, diverse mechanical topics and technics have
been presented including biomechanics, nanomechanics and computational fluid
dynamics, these applications have proven that mechanics has a determinant role
in almost emergent findings and inventions in medicine and biology.
As perspectives, as a mechanical scholar
conducting researchers in nanomechanics and thermal engineering, some case
studies in medicine and biology will be elaborated including CFD simulations,
nanofluids and carbon nanotubes applications in collaboration with biologist
and biomedical scholars.
7. Declaration of conflicts
No conflict
to declare.
8. Authors’ biography
No
biography
9. References
[1] Abreu E. Review of “Basic
Orthopaedic Biomechanics and Mechano-Biology” 3rd Edition, by Van C. Mow and
Rik Huiskes. BioMedical Engineering OnLine. 2005;4:28.
doi:10.1186/1475-925X-4-28.
[2]
http://www.ozeninc.com
[Internet]. Northern California: Ozen Engineering and
ANSYS; [cited 2017 Sep 05]. Available from:
http://www.ozeninc.com/industry-solutions/medical-devices/
[3] Belhadj. A., Boukhalfa.
A., S.A. Belalia, Carbon nanotube structure vibration based on non-local
elasticity, Journal of Modern Materials., Vol.3, No.1, pp.9-13.2017.
[4] Belhadj. A., Boukhalfa.
A., S.A. Belalia, Free vibration modelling of single-walled carbon nanotubes
using the differential quadrature method, Mathematical Modelling of Engineering
Problems, vol. 4 (1), pp. 33-37, 2017.
[5] Ferlay J, Soerjomataram
I, Ervik M, Dikshit R, Eser S, Mathers C et al. GLOBOCAN 2012 v1.0, Cancer
Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France:
International Agency for Research on Cancer; 2013.
[6] Sanginario, A., Miccoli,
B., & Demarchi, D. (2017). Carbon Nanotubes as an Effective Opportunity for
Cancer Diagnosis and Treatment. Biosensors, 7(1),
9.
[7] Elhissi, A., Ahmed, W.,
Hassan, I. U., Dhanak, V. R., D'Emanuele, A. (2011). Carbon nanotubes in cancer
therapy and drug delivery. Journal of drug
delivery, Volume 2012, Article ID 837327, 10 pages, doi:10.1155/2012/8373272012.
[8] Magnetic functionalised
carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Yang
F, Jin C, Yang D, Jiang Y, Li J, Di Y, Hu J, Wang C, Ni Q, Fu D Eur J Cancer. 2011 Aug; 47(12):1873-82.
[9] Yang L, Ng KY, Lillehei
KO , Cell-mediated immunotherapy: a new
approach to the treatment of malignant glioma.Cancer Control. 2003 Mar-Apr; 10(2):138-47.
[10] Dhar S, Liu Z, Thomale J,
Dai H, Lippard SJ. Targeted Single Wall Carbon Nanotube Mediated Pt(IV) Prodrug
Delivery Using Folate as a Homing Device. Journal
of the American Chemical Society. 2008;130(34):11467-11476.
[11] Ruggiero A, Villa CH,
Holland JP, Sprinkle SR, May C, Lewis JS, Scheinberg DA, McDevitt MR, Imaging and treating tumor vasculature with
targeted radiolabeled carbon nanotubes. Int J
Nanomedicine. 2010 oct, vol 2010 (5) :783-802. doi.org/10.2147/IJN.S13300
[12] Son, K. H., Hong, J. H., & Lee, J. W. (2016).
Carbon nanotubes as
cancer therapeutic carriers and mediators. International
journal of nanomedicine, vol.2016(11) : 5163—5185.
doi.org/10.2147/IJN.S112660
[13] Fan, H., Zhang, I. Y., Chen,
X., Zhang, L., Wang, H., da Fonseca, A. C. C., ... & Badie, B. (2012). Intracerebral CpG immunotherapy with carbon nanotubes
abrogates growth of subcutaneous melanomas in mice. Clinical
Cancer Research, 2012,18 (20):5628-5638. doi:10.1158/1078-0432.CCR-12-1911
[14] Fadel TR, Sharp FA, Vudattu N, et al. A carbon
nanotube–polymer composite for T-cell therapy. Nat Nanotechnol.
2014;9(8):639–647.
[15] Meng J, Yang M, Jia F, Kong H, Zhang W, Wang C,
Xing J, Xie S, Xu H? Subcutaneous injection of water-soluble multi-walled
carbon nanotubes in tumor-bearing mice boosts the host immune activity
Nanotechnology. 2010 Apr 9;
21(14):145104.
[16] Villa CH, Dao T, Ahearn
I, Fehrenbacher N, Casey E, Rey DA, Korontsvit T, Zakhaleva V, Batt CA, Philips
MR, Scheinberg D, Single-walled carbon nanotubes deliver peptide antigen into
dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano. 2011 Jul 26; 5(7):5300-11.
[17] Sacchetti C, Rapini N,
Magrini A, Cirelli E, Bellucci S, Mattei M, Rosato N, Bottini N, Bottini
M, In vivo targeting of intratumor
regulatory T cells using PEG-modified single-walled carbon nanotubes. Bioconjug Chem. 2013 Jun 19; 24(6):852-8.
[18] Congressional Research
Service Report Life expectancy in the United States. Mar, 2005. Available at:
http://www.cnie.org/nle/crsreports/05mar/RL32792.pdf. Accessed
[19] Bartlett JG, Gilbert DN, Spellberg B, Seven ways to preserve the miracle of
antibiotics, Clin Infect Dis. 2013 May; 56(10):1445-50.
[20] Dai, X., Guo, Q., Zhao, Y.,
Zhang, P., Zhang, T., Zhang, X., & Li, C. (2016). Functional Silver Nanoparticle as a Benign
Antimicrobial Agent That Eradicates Antibiotic-Resistant Bacteria and Promotes
Wound Healing. ACS applied materials
& interfaces, 8(39), 25798-25807.
[21] Rose. Y, Mattix. B, Rao.A,
and Alexis. F, “Carbon nanotubes and
infectious diseases,” in Nanomedicine in Health and Disease, R. J. Hunter, Ed.,
pp. 249–267, Science Publishers, London, UK, 2011.
[22] L. Jiang, T. Liu, H. He,
Pham-Huy LA, Li L, Pham-Huy C, Xiao D, “Adsorption behavior of pazufloxacin
mesilate on amino-functionalized carbon nanotubes,” Journal of Nanoscience and
Nanotechnology, 2012,12(9) 7271-9.
[23] Wang, W. L., Wu, Q. Y.,
Wang, Z. M., Niu, L. X., Wang, C., Sun, M. C., & Hu, H. Y. (2015). Adsorption removal of antiviral drug oseltamivir and
its metabolite oseltamivir carboxylate by carbon nanotubes: Effects of carbon
nanotube properties and media. Journal of
environmental management, 162 (octobre) : 326-333.
doi.org/10.1016/j.jenvman.2015.07.043
[24] Mehta, A. (2011). Genetic
disorders and hereditary disorders. Retrieved June 10th.
pharmaxchange.info/notes/clinical/genetic_disorders.pdf
[25] Ibraheem, D., Elaissari, A., & Fessi, H.
(2014). Gene therapy and DNA
delivery systems. International journal of
pharmaceutics, 459(1), 70-83.
[26] Ramos-Perez, V., Cifuentes,
A., Coronas, N., de Pablo, A., & Borrós, S. (2013). Modification of carbon nanotubes for gene delivery
vectors. Nanomaterial Interfaces in Biology: Methods and Protocols, 261-268.
[27] Singh R, Pantarotto D,
McCarthy D, Chaloin O, Hoebeke J, Partidos CD, Briand JP, Prato M, Bianco A,
Kostarelos K J Am Chem Soc. 2005 Mar 30; 127(12):4388-96.
[28] Pantarotto D, Singh R,
McCarthy D, Erhardt M, Briand JP, Prato M, Kostarelos K, Bianco A, Functionalized carbon nanotubes for plasmid
DNA gene delivery. Angew Chem Int Ed Engl.
2004 Oct 4; 43(39):5242-6.
[29] Saadeh Y, Vyas D.
Nanorobotic Applications in Medicine: Current Proposals and Designs. American journal of robotic surgery. 2014;1(1):4-11.
doi:10.1166/ajrs.2014.1010.
[30] Varadan VK, Chen LF, Xie
J. Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors
and Nanosystems. Wiley; 2008.
[31] Ceyhan B, Alhorn P, Lang C,
Schuler D, Niemeyer CM. Semisynthetic
biogenic magnetosome nanoparticles for the detection of proteins and nucleic
acids. Small. 2006;2(11) DOI:10.1002/smll.200600282
[32] Murphy D., Challacombe
B., Nedas T., Elhage O., Althoefer K., Seneviratne L., Dasgupta P. Equipment
and technology in robotics. Arch. Esp. Urol.
2007;60(4):349–354.
[33] Ikeda S., Arai F., Fukuda T., Kim E.H., Negoro
M., Irie K., Takahashi I. IEEE Int. Conf.
Intell. Robot. Syst. Edmonton, Canada: 2005. Aug. In vitro patient-tailored
anatomical model of cerebral artery for evaluating medical robots and systems
for intravascular neurosurgery; pp. 1558–1563.
[34] Fann J.I., Goar F.G.S.,
Komtebedde J., Oz M.C., Block P.C., Foster E., Butany J., Feldman T., Burdon
T.A. Beating heart catheter-based edge-to-edge mitral valve procedure in a
porcine model: efficacy and healing response. Circulation.
2004;110(8):988–993.
[35] Nowlin W.C., Guthart
G.S., Younge R.G., Cooper T.G., Gerbi C., Blumenkranz S.J., Hoornaert D.F. Grip
strength with tactile feedback for robotic surgery. 6879880US.
Apr. 2005.
[36] Cuschieri A. Laparoscopic
surgery: current status, issues and future developments. Surgeon.
2005;3(3):125–138.
[37] Freitas R.A., Jr.
Nanotechnology, Nanomedicine and Nanosurgery. Int. J. Surg. 2005;3(12):1–4.
[38] Patel G.M., Patel G.C.,
Patel R.B., Patel J.K., Patel M. Nanorobot: a versatile tool in nanomedicine. J. Drug Target. 2006;14(2):63–67.
[39] C. Darkin, Nanorobots
With Human Embryo,
https://fineartamerica.com/featured/nanorobots-with-human-embryo-christian-darkin.html
[40] Jaiswal, A., H., Thakar,
B., Atanukumar, T., Krunali, and D. B., Meshram, “Nanotechnology revolution:
respirocytes and its application in life sciences” Innovare journal of life
sciences, 1 (1): 8-13, 2013.
[41] Freitas. RA. Jr, Exploratory
design in medical nanotechnology: a mechanical artificial red cell. Artif Cells
Blood Substit Immobil Biotechnol. 1998 Jul; 26(4):411-30.
[42] Hassouna. HI, Blood
stasis, thrombosis and fibrinolysis, Hematol Oncol Clin North Am. 2000 Apr;
14(2):xvii-xxii.
[43] Cavalcanti A, Shirinzadeh
B, Fukuda T, Ikeda S. Nanorobot for brain aneurysm. The International Journal
of Robotics Research. 2009;28(4) : 558-570.
[44] Whitesides. GM,The
'right' size in nanobiotechnology.Whitesides GM.Nat Biotechnol. 2003 Oct; 21(10):1161-5.
[45] Uddin, M. J., Al Kalbani, K.
S., Rahman, M. M., Alam, M. S., Al-Salti, N., & Eltayeb, I. A. (2016). Fundamentals of nanofluids: evolution, applications
and new theory. Journal of Biomathematics and Systems Biology, 2(1) :1-32.
[46] Wang S, Mamedova N, Kotov
NA, Chen W, Studer J. Antigen/antibody immunocomplex from CdTe nanoparticle
bioconjugates. Nano Letters. 2002; 2(8):817–822.
[47] Mah C, Zolotukhin I,
Fraites TJ, Dobson J, Batich C, Byrne BJ. Microsphere-mediated delivery of
recombinant AAV vectors in vitro and in vivo. Mol Therapy, 2000;1(5):
S239–S242.
DOI:
http://dx.doi.org/10.1006/mthe.2000.0174
[48] Edelstein RL, Tamanaha
CR, Sheehan PE, Miller MM, Baselt DR, Whitman LJ, Colton RJ Biosens
Bioelectron. 2000 Jan; 14(10-11):805-13.
[49] Nam JM, Thaxton CS,
Mirkin CA , Nanoparticle-based bio-bar codes for the ultrasensitive detection
of proteins. Science. 2003 Sep 26;
301(5641):1884-6.
[50] Mahtab R, Rogers JP,
Murphy CJ. Protein-sized quantum dot luminescence can distinguish between
"straight", "bent", and "kinked"
oligonucleotides. J Am Chem Soc. 1995; 117(35):9099–9100.
[51] De la Isla A, Brostow W,
Bujard B, Estevez M, Rodriguez JR, Vargas S, Castano VM. Nanohybrid scratch resistant coating for teeth and
bone viscoelasticity manifested in tribology. Mat
Resr Innovat. 2003;7(2):110–114.
[52] Salata, O. V. (2004).
Applications of nanoparticles in biology and medicine. Journal of nanobiotechnology, 2(1), 3.
[53] Ventrelli, L., Ricotti, L., Menciassi, A.,
Mazzolai, B., & Mattoli, V. (2013). Nanoscaffolds
for guided cardiac repair: the new therapeutic challenge of regenerative
medicine. Journal of Nanomaterials,
2013, Volume 2013, Article ID 108485, 16 pages.
http://dx.doi.org/10.1155/2013/108485
[54] Songvorawit, N.,
Tuitemwong, P., Tuchinda, K., & Tuitemwong, K. Fluorescent Dye-Doped Silica
Nanoparticles with Polyclonal Antibodies for the Rapid Detection of Salmonella
spp. Chiang Mai University Journal of Natural, 2013 ; 12(1):25-33.
[55] Costescu, A., Ciobanu, C.
S., Iconaru, S. L., Ghita, R. V., Chifiriuc, C. M., Marutescu, L. G., &
Predoi, D. (2013). Fabrication,
characterization, and antimicrobial activity, evaluation of low silver
concentrations in silver-doped hydroxyapatite nanoparticles. Journal of Nanomaterials, Volume 2013, Article ID 194854, 9 pages.
http://dx.doi.org/10.1155/2013/194854
[56] Ferrua M, Singh R.
Modeling the Fluid Dynamics in a Human Stomach to Gain Insight of Food
Digestion. Journal of Food Science. 2010;75(7):R151-R162.
[57] De Leval. M. D et al.
,Use of computational fluid dynamics in the design of surgical procedures:
Application to the study of competitive flows in cavopulmonary connections, The
Journal of Thoracic and Cardiovascular Surgery, 1996, 111( 3), pp. 502-513.
[58] Rispoli VC, Nielsen JF,
Nayak KS, Carvalho JLA. Computational fluid dynamics simulations of blood flow
regularized by 3D phase contrast MRI. BioMedical
Engineering OnLine. 2015;14(110) :1-23. DOI 10.1186/s12938-015-0104-7
[59]
Doost
SN, Ghista D, Su B, Zhong L, Morsi YS. Heart blood flow simulation: a
perspective review. BioMedical Engineering OnLine.
2016;15(1):101. doi:10.1186/s12938-016-0224-8.
[60] R. F. Rickard, J. Wilson
and D. A. Hudson, Characterization of a rodent model for the study of arterial
microanastomoses with size discrepancy (small-to-large),Laboratory Animals,
vol. 43, no. 4, pp. 350-356, 2009.